- Luthra & Luthra
advertisement

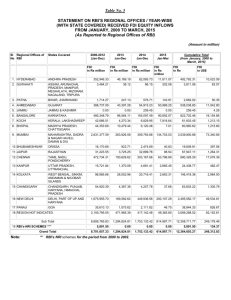
INBOUND M&A IN INDIA – OPPORTUNITIES AND CHALLENGES Investing in Emerging Markets 2011 The Harvard Club, New York City Mohit Saraf Senior Partner Luthra & Luthra Law Offices TODAY’S SCENARIO For years, US has been the biggest investor in India Economic Downturn in the US Liquidity Crunch High Degree of Uncertainty Increasing Inter linkages in Global Economy Corporate Governance Issues in the India and the US Retreating Private Equity 29 November 2011 Slide 2 FUNDAMENTALS HAVE NOT CHANGED Synergies : Often 2+2 > 4 Access to new markets, established customer bases and supply chains Access to technology Brand equity and diversification Economies of scale Consolidation 29 November 2011 Slide 3 ISN’T THIS THE RIGHT TIME…….. Easing Valuation Multiples Lower Competition from Private Equity Today Sellers Often Lack Bargaining Power; Forced Exits Undeployed Cash in a Falling Interest Regime: Helpful in Stimulation of Growth and Expansion of Margins Long Term Value Driver: Capturing Synergy Benefits 29 November 2011 Slide 4 MARKET CHALLENGES Volatile Market : mark to market losses Mismatch of Expectations : seller expecting valuation to go north and buyer expecting valuation to go south Raising of third party capital much more challenging Leveraged Companies becoming soft targets 29 November 2011 Slide 5 DEAL MAKING TODAY Target Identification: Lots on the block Comprehensive Due Diligence : Like never before Representations and Warranties: A challenge Deal Cancellation Ability: Strong reluctance Conditions Precedent Material Adverse Effect Clauses Purchase Price Adjustments in Listed Entities 29 November 2011 Slide 6 REGULATORY CHANGES IMPACTING M&A Forward looking reforms: New Takeover Code (2011). Competition Commission of India (“CCI”) approval required for M&A (2011). Removal of prior approval under Press Note 1 of 2005 (2011). Liberalization in the escrow regime for non-residents (2011). Certain share transfers no longer require RBI approval (2011). In–principle approval for FDI in multi brand retail and for increase in FDI cap in single brand retail (2011). 13 April, 2015 Slide 7 REGULATORY CHANGES IMPACTING M&A Negative changes: FDI in the pharmaceutical sector requires approval (2011). FDI in instruments with built-in options to be considered as debt. (This has now been deleted) (2011). 13 April, 2015 Tax liability issues eg. Vodafone case. Slide 8 FORWARD LOOKING REFORMS 13 April, 2015 Slide 9 INTRODUCTION OF THE NEW TAKEOVER CODE Salient features: Threshold limit for open offer trigger increased from 15% to 25%. This enables acquisition of a higher stake without having to comply with the open offer process. Minimum open offer size increased to 26% from 20%. This provides an exit to higher number of shareholders. Non-compete fee to now be part of offer price. This may increase cost of acquisitions. 13 April, 2015 Slide 10 CCI TO APPROVE OF ‘COMBINATIONS’ Any M&A deal where the aggregate value of assets or turnover of the individual entity or the group exceeds certain thresholds specified in the Competition Act would trigger a filing requirement with the CCI. The CCI must then determine whether the combination causes or is likely to cause an appreciable adverse effect on competition within the relevant market in India. The transaction can be consummated after the expiry of 210 days of the filing being made with the CCI or the grant of approval by the CCI, whichever is earlier. 13 April, 2015 Slide 11 FDI IN MULTI BRAND RETAIL In–principle approval has been granted for FDI in multi-brand retail upto 51% under the approval route. This is subject to, inter alia, the following conditions: Minimum amount to be brought in by the foreign investor to be USD 100 million. At least 50% of the total FDI must be invested in back-end infrastructure (includes capital expenditure on all activities, excluding front-end units. Excludes expenditure on land cost and rentals). 30% procurement of manufactured/ processed products must be from SMEs. Government to have first right on procurement of agricultural products. 13 April, 2015 Slide 12 FDI IN SINGLE BRAND RETAIL In-principle approval granted for increase in FDI in single brand retail from 51% to 100% under the approval route. This is subject to, inter alia, the following conditions: Products to be sold under the same brand internationally. Foreign investor must be the owner of the brand. Single brand retail would cover only products branded during manufacture. 13 April, 2015 For FDI above 51%, 30% sourcing must be from SMEs. Slide 13 OTHER POSITIVE REFORMS Removal of Press Note 1 of 2005: Earlier, prior FIPB approval was required where the foreign investor had an existing joint venture, technology transfer or trade mark agreement in the same. Typically an NoC from the existing Indian partner was required. This has now been removed. Liberalization in escrow regime: To facilitate FDI transactions, it is now permissible to open non-interest bearing escrow accounts without RBI approval, subject to certain specified conditions. The escrow account can remain operational for a maximum of 6 months. Transfer of shares without RBI approval: Certain share transfers from a resident to a non-resident can now occur without RBI approval, subject to certain specified conditions eg. where FIPB approval has been obtained, or where the pricing norms under FEMA have not been followed provided FDI policy and FEMA (in terms of sectoral caps and conditionalities) are complied with. 13 April, 2015 Slide 14 NEGATIVE REFORMS 13 April, 2015 Slide 15 FDI IN THE PHARMACEUTICAL SECTOR Earlier, 100% FDI was permitted in the pharmaceutical sector without any Government approval being required. This has been changed as follows: FDI upto 100% is permitted for greenfield investments in the pharmaceuticals sector, without approval. FDI upto 100% is permitted for brownfield investments (i.e. investments in existing companies), under the Government approval route. This is proposed to be reviewed in another six months as it is contemplated that, by then, the CCI would be able to effectively monitor ‘combinations’ in the pharma sector. 13 April, 2015 Slide 16 FDI IN INSTRUMENTS WITH BUILT-IN OPTIONS The FDI Policy issued in 2011 carried the following change: Only equity shares, fully, mandatorily and compulsorily convertible debentures and preference shares with no built-in options of any kind would be eligible instruments for FDI. Equity instruments issued/transferred to non-residents having inbuilt options to be treated as debt and have to comply with the extant guidelines on external commercial borrowings. This has now been deleted. However, the RBI has had concerns regarding such exit mechanisms where non-residents have a guaranteed rate of return. Thus, it is quite likely that RBI may notify that FDI in such instruments would not be permissible. 13 April, 2015 Slide 17 CAPITAL GAINS TAX ISSUES: VODAFONE CASE Bona fide business purpose v. sham, fraudulent and colourable transaction. No tax treaty benefit available (Cayman companies). Non-compete fee was being paid to Essar. Undertaking to discontinue operations in India. FIPB disclosure. 13 April, 2015 Slide 18 THANK YOU Mohit Saraf Senior Partner, Luthra & Luthra Law Offices Mumbai | New Delhi | Bangalore Email : msaraf@luthra.com 29 November 2011 Slide 19