view - The George Washington University
advertisement
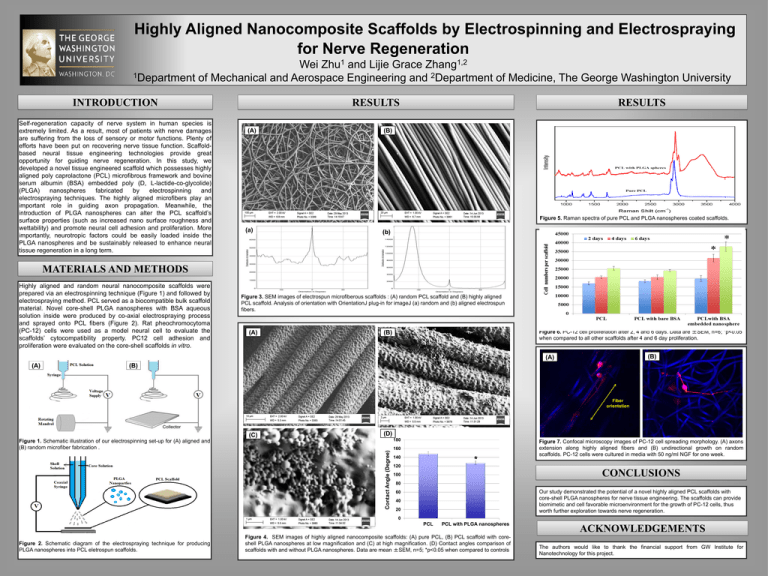
Highly Aligned Nanocomposite Scaffolds by Electrospinning and Electrospraying for Nerve Regeneration 1 Zhu 1,2 Zhang Wei and Lijie Grace 1Department of Mechanical and Aerospace Engineering and 2Department of Medicine, The George Washington University INTRODUCTION Self-regeneration capacity of nerve system in human species is extremely limited. As a result, most of patients with nerve damages are suffering from the loss of sensory or motor functions. Plenty of efforts have been put on recovering nerve tissue function. Scaffoldbased neural tissue engineering technologies provide great opportunity for guiding nerve regeneration. In this study, we developed a novel tissue engineered scaffold which possesses highly aligned poly caprolactone (PCL) microfibrous framework and bovine serum albumin (BSA) embedded poly (D, L-lactide-co-glycolide) (PLGA) nanospheres fabricated by electrospinning and electrospraying techniques. The highly aligned microfibers play an important role in guiding axon propagation. Meanwhile, the introduction of PLGA nanospheres can alter the PCL scaffold’s surface properties (such as increased nano surface roughness and wettability) and promote neural cell adhesion and proliferation. More importantly, neurotropic factors could be easily loaded inside the PLGA nanospheres and be sustainably released to enhance neural tissue regeneration in a long term. RESULTS RESULTS (A) (B) Figure 5. Raman spectra of pure PCL and PLGA nanospheres coated scaffolds. (a) (b) MATERIALS AND METHODS Highly aligned and random neural nanocomposite scaffolds were prepared via an electrospinning technique (Figure 1) and followed by electrospraying method. PCL served as a biocompatible bulk scaffold material. Novel core-shell PLGA nanospheres with BSA aqueous solution inside were produced by co-axial electrospraying process and sprayed onto PCL fibers (Figure 2). Rat pheochromocytoma (PC-12) cells were used as a model neural cell to evaluate the scaffolds’ cytocompatibility property. PC12 cell adhesion and proliferation were evaluated on the core-shell scaffolds in vitro. Figure 3. SEM images of electrospun microfiberous scaffolds : (A) random PCL scaffold and (B) highly aligned PCL scaffold. Analysis of orientation with OrientationJ plug-in for imageJ (a) random and (b) aligned electrospun fibers. (A) Figure 6. PC-12 cell proliferation after 2, 4 and 6 days. Data are ±SEM, n=6; *p<0.05 when compared to all other scaffolds after 4 and 6 day proliferation. (B) (B) (A) (A) (B) Fiber orientation (C) (D) 180 Contact Angle (Degree) Figure 1. Schematic illustration of our electrospinning set-up for (A) aligned and (B) random microfiber fabrication . 160 140 * 120 Figure 7. Confocal microscopy images of PC-12 cell spreading morphology. (A) axons extension along highly aligned fibers and (B) undirectional growth on random scaffolds. PC-12 cells were cultured in media with 50 ng/ml NGF for one week. CONCLUSIONS 100 80 Our study demonstrated the potential of a novel highly aligned PCL scaffolds with core-shell PLGA nanospheres for nerve tissue engineering. The scaffolds can provide biomimetic and cell favorable microenvironment for the growth of PC-12 cells, thus worth further exploration towards nerve regeneration. 60 40 20 0 PCL Figure 2. Schematic diagram of the electrospraying technique for producing PLGA nanospheres into PCL eletrospun scaffolds. PCL with PLGA nanospheres Figure 4. SEM images of highly aligned nanocomposite scaffolds: (A) pure PCL, (B) PCL scaffold with coreshell PLGA nanospheres at low magnification and (C) at high magnification. (D) Contact angles comparison of scaffolds with and without PLGA nanospheres. Data are mean ±SEM, n=5; *p<0.05 when compared to controls ACKNOWLEDGEMENTS The authors would like to thank the financial support from GW Institute for Nanotechnology for this project.