- ePrints Soton

Joint Academic Research Programme for Defence
Microfluidic devices for structural health monitoring: Part 1
Overview
Dstl and EPSRC have jointly funded a 3-year project at the University of Southampton to develop corrosion detection and monitoring systems for land, marine and aerospace structures under grant number EP/F004362/1.
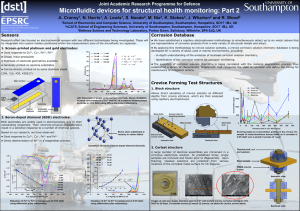
By combining microfluidics with new, emerging sensor technologies, this project aims to develop in-situ systems that not only detect the onset of structural corrosion, but can also assess its state and apply appropriate remediation to inhibit its progress. The principle is illustrated in the schematic shown below.
Knowledgebase
Sensors
Pump
Microprocessor
Instrumentation and HV control
Microfluidic ion separator
Non-return valve Fluidic systems
Electronic systems
Remediation system
A. Cranny
1
, N. Harris
1
, A. Lewis
1
, S. Neodo
2
, M. Nie
2
, K. Stokes
3
, J. Wharton
2
and R. Wood
2
1
School of Electronics and Computer Science, University of Southampton, Southampton, Hampshire, SO17 1BJ, UK
2
nCATS, School of Engineering Sciences, University of Southampton, Southampton, Hampshire, SO17 1BJ, UK
3
Defence Science and Technology Laboratory, Porton Down, Salisbury, Wiltshire, SP4 0JQ, UK
Microfluidics
Pump control
Fluid sampler
Calibration solutions
Buffer reservoir
Monitored structure
For example
The onset or extent or corrosion in a structure can be determined by monitoring changes in the concentrations of specific metal cations in the immediate environment surrounding the structure. A number of sensor technologies can be used to detect these corrosion products, but few (if any) can detect all of them simultaneously over a range of different and potentially hostile environments.
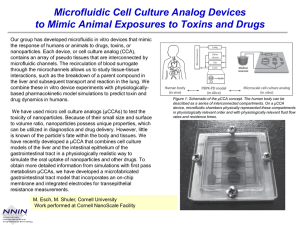
Our strategy is to separate and isolate groups of corrosion products from fluids sampled at a monitored structure and pass them on to appropriate sensor systems for quantification. A novel microfluidic ion separator using capillary electrophoresis
(CE) is being developed for this purpose.
In capillary electrophoresis, ions are separated in an electric field due to differences in their electrical mobilities. In standard
CE, this separation is achieved as ions move along a capillary of typically 1 m in length. The challenge with miniaturisation is to achieve the same objective along a channel of considerably shorter length.
A
C
A
A
B C
D
A
Microfluidic ion separator: Gold electrodes patterned on to a glass wafer. A = Electric field generation electrodes.
B = Zeta potential modification electrode. C = Contactless conductivity detectors. D = Electrochemical sensor.
Schematic showing the basic units of a microfluidic corrosion detection and monitoring system for use in spot measurement or (semi-)permanent installation.
The project is overseen by a steering committee that is comprised of manufacturers and other interested parties that are representative of the defence industry, namely:
● Dstl ●
Rolls
Royce Marine ● GKN Aerostructures ● Lloyd’s Register
A second theme to this research programme is the investigation of how corrosion processes are initiated and evolve in a number of commonly used structural metals, alloys and composites, with particular emphasis on crevice corrosion. This will help in the formulation of scientific models to describe these processes and also help in the development of new remediation strategies.
Objectives
From the science and fabrication technologies developed here, we ultimately hope to see these microfluidic sensor monitoring systems integrated within critical structures during manufacture, as illustrated by the concept of the
‘smart’ pipeline gasket shown to the right.
Such systems will:
● Extend platform life
● Aid scheduling of maintenance cycles
● Augment existing corrosion science knowledge
Our approach is to do this by shuffling the ions back and forth along the channel, thus effectively increasing the travel distance, by alternating the direction of the electroosmotic flow
(EOF): a cohesive flow that transports all particles irrespective of their charge. This is achieved by varying the zeta potential along the channel walls.
Once sufficient separation is achieved between the ion species with the greatest electrophoretic mobility and the remaining ion groups, this species is isolated and transported to the measurement zone for identification and quantification before being extracted to waste.
The whole process is then repeated for the second most mobile species, then the third most, and so on. The decision as to when to reverse the EOF direction and isolate species is made by using contactless conductivity detectors at each end of the channel that monitor the arrival times of ion groups. Hence, the entire process operates as a closed loop control system requiring minimum intervention.
2.4
2.2
2.0
1.8
Cu
2+
Microfluidic ion separator: Separation channel and sample injection channel buried within an insulated PDMS layer.
PDMS layer
Micro-channel
Dissemination
To date, results from this project have been presented at 3 national conferences, 5 international conferences (Australia,
China, France and USA) and published in 12 peer reviewed proceedings and journals.
For more information, visit: http://mdfshm.ecs.soton.ac.uk
The ‘smart’ gasket – example of the integration of microfluidics and sensor systems within a structure.
1.6
1.4
1.2
1.0
Cl
-
0.8
0 100 200 300 400 500 600
Elapsed Time (s)
700 800 900 1000
Example electropherogram demonstrating separation of CuCl
2 in deionised water.
SU8 insulation
Solution reservoir
Glass wafer
Microfluidic ion separator: Cross section and detail showing position of buried separation channel in PDMS layer.