The Nuts and Bolts of Directing and Performing Laboratory Tests in
advertisement

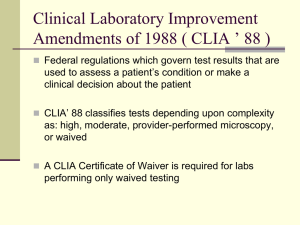
#MS2012 CLW2 The Nuts and Bolts of Directing and Performing Laboratory Tests in Your California Practice Jan Otey, CLS, Examiner II Room: Colton 2 Please no cameras or recorders during the class presentation. You will be asked to leave the room if request is not followed. 1 #MS2012 Make sure to check in and out for each course – you must do this even if you are staying in the same room for multiple courses. Hand in your course ticket as you leave the class in order to receive credit. The ticket is the ONLY way to receive credit for this course. If you must leave the class for any amount of time keep in mind if you are out of the room for more than 10 minutes, you will not receive any CE credit. Please remember to complete the evaluation forms on the back of your course tickets. 2 #MS2012 If you are an Option 1 or Option 3 participant, you have received COA Buck$ to purchase products from participating exhibitors in the Exhibit Hall. Look for the sign in exhibitor booths and use your Buck$! A special thank you goes to our industry sponsors: Vision West, VSP Global, Vistakon, Alcon, Allergan, CooperVision, Luxottica, Abbott Medical Optics for their support of this conference. If you have a cell phone or pager, please turn it off. If you must take a call, do so outside the room. 3 CLW2 – The Nuts and Bolts of Directing and Performing Laboratory Tests in Your California Practice Jan Otey, CLS, Examiner II This course material and information was developed independently of any assistance. I do not have any financial arrangements or affiliations with any corporate organizations (ex. Salary, honorarium, etc.) 4 THE NUTS AND BOLTS OF DIRECTING AND PERFORMING LABORATORY TESTS IN YOUR CALIFORNIA PRACTICE P R E S E N T E D B Y: Jan Otey, CLS, MT (ASCP), Examiner II California Department of Public Health Laboratory Field Services Sunday, November 11, 2012 5 Course Objectives At the conclusion of this program you should understand: What Laboratory Field Service (LFS) does. Why LFS and AB761 may be relevant to your practice. The responsibilities of a Laboratory Director. 6 (Course Objectives cont.) How to successfully apply for a Clinical Laboratory Registration and a CLIA Certificate of Waiver. The most common application problems that delay approval. Waived laboratory inspections. The testing documentation that you would be expected to maintain. 7 Laboratory Field Services Part of California Department of Public Health and the Office of the State Public Health Laboratory Director ◦ License laboratories, laboratory personnel, tissue banks, and blood banks ◦ State laboratory inspections State agent for Center for Medicare and Medicaid Services (CMS) ◦ CLIA laboratory inspections ◦ Responsible for CLIA certification applications 8 Why should I care? California Business & Professions Code Chapter 3 §1241. (a) This chapter applies to all clinical laboratories in California or receiving biological specimens originating in California for the purpose of performing a clinical laboratory test or examination, and to all persons performing clinical laboratory tests or examinations or engaging in clinical laboratory practice in California or on biological specimens originating in California, except as provided in subdivision (b). 9 California Business & Professions Code Changes Passage of new law, AB 761 (Roger Hernandez) effective January 1, 2013 ◦ An act to amend Sections 1206.5, 1209, and 3041 of the Business and Professions Code, relating to optometrists. 10 B&P Code §1206.5 §1206.5. (a) Notwithstanding subdivision (b) of Section 1206 and except as otherwise provided in Section 1241, no person shall perform a clinical laboratory test or examination classified as waived under CLIA unless the clinical laboratory test or examination is performed under the overall operation and administration of the laboratory director, as described in Section 1209, including, but not limited to, documentation by the laboratory director of the adequacy of the qualifications and competency of the personnel, and the test is performed by any of the following persons: (13) A licensed optometrist as authorized under Chapter 7 (commencing with Section 3000). (14) Other health care personnel providing direct patient care. 11 B&P Code §1209 §1209. (a) As used in this chapter, "laboratory director" means any person who is a duly licensed physician and surgeon, or, only for purposes of a clinical laboratory test or examination classified as waived, is a duly licensed naturopathic doctor, or a duly licensed optometrist serving as the director of a laboratory which only performs clinical laboratory tests authorized in paragraph (10) of subdivision (e) of Section 3041 that are classified as waived, or is licensed to direct a clinical laboratory under this chapter and who substantially meets the laboratory director qualifications under CLIA for the type and complexity of tests being offered by the laboratory. 12 B&P Code §3041 (e) An optometrist who is certified to use therapeutic pharmaceutical agents pursuant to Section 3041.3 may also perform all of the following: (9) Ordering of smears, cultures, sensitivities, complete blood count, mycobacterial culture, acid fast stain, urinalysis, tear fluid analysis, and X-rays necessary for the diagnosis of conditions or diseases of the eye or adnexa. An optometrist may order other types of images subject to prior consultation with an ophthalmologist or appropriate physician and surgeon. (10) A clinical laboratory test or examination classified as waived under CLIA and designated as waived in paragraph (9) necessary for the diagnosis of conditions and diseases of the eye or adnexa, or if otherwise specifically authorized by this chapter. 13 January 1, 2013 An optometrist will be able to direct a California waived laboratory in which “waived” tear fluid analysis and urinalysis laboratory tests may be performed by the optometrists. 14 Laboratory Director Responsibilities CBPC ◦ ◦ ◦ ◦ ◦ §1209(b)(1) Overall operation and administration Technical and scientific operation Selection of procedures Reporting of results Compliance with all State and Federal Law 15 Laboratory Director Responsibilities CBPC §1209(b) (1) The laboratory director is responsible for the overall operation and administration of the clinical laboratory, including administering the technical and scientific operation of a clinical laboratory, the selection and supervision of procedures, the reporting of results, and active participation in its operations to the extent necessary to ensure compliance with this act and CLIA. He or she shall be responsible for the proper performance of all laboratory work of all subordinates and shall employ a sufficient number of laboratory personnel with the appropriate education and either experience or training to provide appropriate consultation, properly supervise and accurately perform tests, and report test results in accordance with the personnel qualifications, duties, and responsibilities described in CLIA and this chapter. 16 Director’s Attestation Assume all director responsibilities Held jointly responsible with the owner for any violations of law by this clinical laboratory Any false statements made in obtaining or retaining State registration or CLIA certification may be grounds for revocation Director and the laboratory owner are jointly responsible for notifying LFS of any changes in the laboratory ownership, directorship, name or location within 30 days Director will be responsible as director until CDPH receives a signed statement from you notifying the Department of your resignation. 17 Instructions for Applying for a California Clinical Laboratory Registration and CLIA Certificate of Waiver If you wish to apply for a CLIA Certificate of Waiver , you must also apply for a state registration, not a state license. California Clinical Laboratory Registration Fee and Required Documents: ● Registration fee payable to the State of California Department of Public Health. The registration fee is non-refundable and is subject to change with each fiscal year. The fee schedule may be downloaded from: http://www.cdph.ca.gov/programs/lfs/Documents/A-License-FeeSchedules.pdf ● Federal CLIA program will bill you separately for the CLIA certification fee. ● Original signatures must be on all forms where signatures are required. State forms to complete and submit: ● LAB 155 — Application for Clinical Laboratory Registration ● LAB 116 — Laboratory Personnel Report ● LAB 183 — Director Attestation Federal form to complete and submit: ● CMS 116 — CLIA application, unless already certified by CMS 18 PROBLEMS 19 MAIL New applications ◦ ~120/month Renewals ◦ ~14,000/year Change in Director or Owner Missing or incomplete forms 20 Time Frames Your office to reviewer ~ 1 week State database entry ~ 1 week CLIA database entry < 30 days ◦ Invoice from CMS CLIA number to State ~ 1 week Activation of State Registration ~ 1 week CLIA certificate mailed ~ 1 week after payment received 21 2 of every 5 new applications Application returned and/or incomplete application letter mailed 22 California Registration Errors #4. - Legal name of owner (e.g. corporation name) #6. - Hours per week on-site Signature of Laboratory Director AND Laboratory Owner 23 CLIA Application Errors Name of Director (Laboratory) Type of Certificate not specified Estimated total volume of waived tests Be sure to sign and date application Do NOT submit data in sections: ◦ V – Multiple sites ◦ VII – PPM testing ◦ VIII – Non-waived testing 24 CLIA Application VI - Waived testing (from page 3 of CMS 116 form) Identify the waived testing performed. Be as specific as possible. This includes each analyte test system or device used in the laboratory. e.g. (Osmolarity - TearLab) (Adenovirus - Rapid Pathogen Screening (RPS)) (Adenovirus - Nicox) (Urinalysis - ? Manufacturer) Indicate the estimated total annual test volume for all waived tests performed ________________ 25 Back to square one See slide #20 Time Frames 26 Ready to start testing California Clinical Laboratory Registration CLIA Certificate of Waiver 27 Renewal State Laboratory Registration – 1 year ◦ Expiration date: Renewal forms 75 days prior >30 days + 25% delinquent fee >60 days – termination letter Letter to CLIA to terminate CLIA Certification – 2 years 28 Inspections ? 29 CLIA Certificate of Waiver Texas – 15,773 Florida – 14,958 California – 14,263 30 Inspections ? State / CLIA Routine Validation Complaint 31 TearLab Osmolarity System Documentation of: ◦ Date received, lot number, & exp. date of each shipment of test cards ◦ Date & Electronic Check Card test results each day of use ◦ Date and external control results for Each new test card shipment Each new test card lot number Monthly to check storage 32 RPS - AdenoPlus Documentation of: ◦ Date received, lot number, & exp. date of each shipment of test cassettes ◦ Date of external control results Each new shipment Each new lot number Once by each new untrained operator prior to patient testing 33 In Conclusion A request from our program techs who will be processing your applications: Please include a cover letter designating the best person to call or e-mail, including their direct line and e-mail address, if clarification of your application is needed. 34 Contact Information California Department of Public Health Laboratory Field Services 850 Marina Bay Parkway Richmond, CA 94804-6403 Jan Otey, Examiner II (510) 620-3816 Fax (510) 620-3688 E-mail: jotey@cdph.ca.gov 35 QUESTIONS 36