Reed Presentation - MFRPA 2014 - Home
advertisement

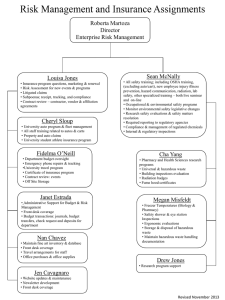
Standard 2 Breakout Session Building a State Training Plan Mark M. Reed, R.S., MPA, MPH Branch Manager Kentucky Department for Public Health Food Safety Branch March 11, 2014 First Things First…Greetings from the Bluegrass State! Background: KY DPH Food Manufacturing Section Natasha Collins (502) 484-3412 Food Manufacturing Inspector Areas • Some 1100 Firms +/• Team of 5 (plus 1) Field Inspectors • Small Program Jay Fillman (270) 684-2047 Paul Rice (606) 638-0770 Raquel Rouse (502) 382-6312 Annhall Norris (859) 236-8159 Initial Self-Assessment – Standard 2 Purpose –To Build a Strong Foundation From the Ground UP • • • From Self-Assessment • – Good Job Capturing Staff Training, but – No formal, written, standardized plan To establish formal Food Safety Branch administrative policy regarding the training plan/pathway for Food Manufacturing Section Staff To ensure that all inspectors receive basic and advanced food inspection training as well as continued training and education in order to adequately perform their work assignments and continue to develop professionally Highly Trained and competent inspection team essential in building a solid foundation for a highly functional and strong food protection program Staff Training and Development Plan— Level I Web Courses • FDA ORA-U Courses: – Complete Registration w/in 30 days from employment – Complete Manufactured Foods Curriculum w/in 90 days of employment (some 30+ courses) – Forward Certificates/Transcript to Designated Training and Compliance Officer • FEMA Incident Command: Complete w/in 180 days from employment: – IS-100.b Introduction to Incident Command System – IS-200.b ICS for Single Resources and Initial Action Incidents – IS-700.a National Incident Management System (NIMS)An Introduction – IS-800.b National Response Framework – An Introduction Staff Training and Development Plan — Level I Web Courses • FDA Food Defense 101 – Food Defense Awareness for the Food Professional – Food Defense Awareness for the Front-Line Employee – Food Defense Regulations – ALERT for Owners and Operators of Food Facilities • Complete w/in 180 days of Employment • Certificates forwarded to Designated Training and Compliance Officer Staff Training and Development Plan — Level I Live Classroom Complete w/in 24 Months of Employment: • FD152 Food Processing Technology • FD170 Application of the Basics of Inspection and Investigation • FD180 Application and Evidence Development (Replaces Both 150 and FD151) • Segment One Seafood HACCP Alliance Course and Segment Two Live Training • • *Better Process Control School *AIB International Labeling of FDA Regulated Foods (or Equivalent Course Approved by Management The asterisked (*) courses shall be considered optional where agency travel restrictions or funding limitations are encountered. Escape Clause: It shall be understood that, under extenuating circumstances (including but not limited to class availability, and program funding/travel restrictions), an extension of these time limits may be granted by the employee’s direct line supervisor. Staff Training and Development Plan — Level I Joint/Audit Field Inspections • 10 field-level, Joint/Audit Inspections with Qualified Trainer to be Completed w/in 18 Months of Employment • Field Inspector Must Receive at least Two Acceptable Evaluations • If Competency is Demonstrated, Lower Number than 10 Inspections May be Completed (Must Still Receive Two Acceptable Evaluations) • Field Training to be Captured on Appropriate Forms and Submitted to Designated Training and Compliance Officer • Successful Completion of this Requirement, Along with Level I On-Line and Live Trainings Qualifies Inspector for GMP (nonSpecialty Area Inspections) Level II Advanced (Specialty Area) Food Inspection Classroom Training To Be Completed w/in 36 Months of Employment: • FD202 Conducting Acidified Food Inspections • FD304 Low Acid Canned Food Inspections (*Replaces FD203) • FD203 Conducting Low Acid Canned Food Inspections • FD219 Juice HACCP and Conducting Juice Inspections • FD249 Conducting Seafood Inspections Additionally, FDA StateStandardized Shellfish Officer (SSO) and Shellfish Program Field Inspectors to Complete: • FD245 Shellfish Plant Standardization (*Replaces both FD140 Basic Shellfish Plant Sanitation and FD241 Shellfish State Standardization Officer) Level II “Specialty Area” Joint Field Inspection/Audit Inspections To be Completed Within an Additional 18 Months and Prior to Independently Conducting an Inspection of a “Specialty Area” Plant: • Successful Completion of each “Specialty Area” Live Training • Field Inspector to have Conducted Minimum of 3 Joint Inspections of Each “Specialty Area” firm with Qualified Trainer • Field Inspector Shall Have Received a Total of 2 Successful Evaluations for each “Specialty Area” firm. • OTJ Training Inspections to be Properly Documented and Submitted to Training & Compliance Officer “Escape Clause” for Experienced Inspection Staff • Signed Affidavit Utilized to Affirm Inspector’s Experienced inspectors hired Competence to Conduct Initial before KY Food Safety Branch (GMP) and “Specialty Area” enrollment in MFRPS (2011) will Inspections be granted an exemption from “initial” and “specialty area” field • Does Not Eliminate training provided they remain Requirement for Standard 4 proficient in their inspectional and Field Inspection Audits documentation duties… • No Exemption for Web-Based or Live Classroom Training Qualified Trainer… • Successfully Completed KY Definition: Any Food Advanced Food Inspection Manufacturing Section Inspector Training Coursework and Field (or Qualified FDA Inspector) Training in Any Areas Where Recognized by the Food Program the Trainer Performs Advanced Manager as Having Field Training Experience and Communication Skills Necessary to Train Other • See MFRPS Interpretation Investigators, and Who Has: • Demonstrated Competency for Basic food Inspection Training to the Food Program Manager (or FDA District Office); and Level III Advanced (Response) Training To Be Completed in a Timely Manner, as Class Space and Funding are Available: • ER220 Traceback Investigations • ER321 Produce Farm Investigations • FD325 Foodborne Illness Investigations • PER-273 NCBRT (LSU) A Coordinated Response to Food Emergencies • PER-298 NCBRT (LSU) Team Approach to Foodborne Outbreak Response And Just When You Think You’ve Reached the Finish Line.. Continuing Education and Training Requirements: • Each Manufactured Foods Inspector to Complete a Minimum of 36 Contact Hours of Classroom/Continuing Education Every 36 Months • Mostly Satisfied by KY “Registered Sanitarian” Requirement of 10 Contact Hours Per Year • R.S. Renewal Card to Training and Compliance Officer • In Reality No Finish Line • Continuous Learning & Professional Development Examples of Continuing Education and Training • Completion of Additional Web-Based or Live Training Courses • Attendance at Professional Association Meetings/Conferences – KAMFES – AFDOSS – AFDO – NEHA • In-House Staff Development and Training IMPORTANT: Maintain Agendas, Certificates, and other Proof of Attendance Training Records and Documentation • Affidavits for • Training and Compliance Courses/Continuing Education Officer’s Responsibility to: with Missing Documentation – Maintain History of Training • Each Employee Responsible Provided to Each Inspector for Promptly Forwarding to Designated Training and – Maintain Pertinent Training Compliance Officer: Records for Each Inspector – Agendas – Certificates of Completion – Joint Inspection Documentation – Certificates of Attendance – Current R.S. Renewal Certification that Document Completion of Standard 2 Staff Included in Training Policy • Policy Applies to Food Manufacturing Section Field Staff Whose Job Duties Include: – Federal/State Food contract Inspections and/or – State-Level Inspections of Food Processing, Storage or Distribution Firms • Section Supervisor to Complete all Online/Live Classroom and Continuing Education Provisions (Exempt From Field Training) Failure to Comply • Consider Adding FTC Clause May Result In: • Reassignment of Employee • Ineligibility for FDA-Sponsored Training • Other Disciplinary Action in Accordance with Office of Human Resource Management (OHRM) Provisions, Depending Upon Circumstances “Hardship Clause” Meant to Address: • Employee Fiscal Hardship (Up Front Expenses, Hotel, Per Diem, etc.) – “Company Card,” etc. • Other Employee Issues (Family, Medical), etc. It is understood that in situations where an employee’s up-front expenses (mileage, meals, lodging), prior to agency reimbursement, would create a severe economic hardship on the employee, exceptions may be made to this policy, including the granting of additional time to complete live training requirements. Such situations will be evaluated on a case-by-case basis by the employee’s direct line supervisor and Branch Manager… Considerations & Lessons Learned (In No Particular Order) • • • • • • • • • • • • Beg, Borrow or “Steal” from Colleagues Expect Multiple DRAFTS, Reviews Revisions Must Have Leadership Buy-In Must be Sustainable Include Time Frames Exceptions for Seasoned Staff Where are Records Maintained Who is Responsible for Training and Continuing Educational Records How Will Records Be Maintained (Hard Copy or Electronic) Compile Course Descriptions Expiration Date on Courses Course Pre-Requisite Challenges • • • Will Plan Create a Career Ladder for Employee Advancement/Can It Include Hyperlinks in Training Plan PHAB Accreditation Documentation MFRPS Requirements • Written, Documented Training Plan – We’ve Got One Now! • Plan Implemented – Effective January 1, 2014 – Ask Us Next Year How Well Its Working – Some Recalibration or Adjustments May be in Order Contact Information Mark M. Reed, R.S., MPA, MPH Branch Manager Kentucky Department for Public Health Food Safety Branch Email: Mark.Reed@ky.gov Phone: (502) 564-7181