Health and Safety in the Laboratory
advertisement

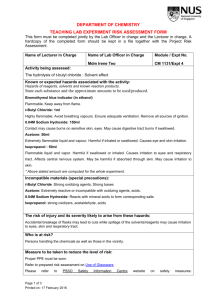
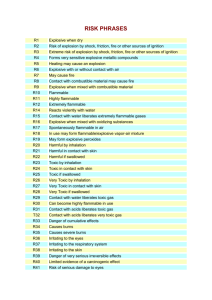
Health and Safety in the Laboratory I. Introduction Law Protecting Lab Employees “Occupational Exposures to Hazardous Chemicals in the Laboratory” Requires: Training Appropriate MSDS Safety Equipment II. What is a Hazardous Chemical? Determined to be cancer-causing, toxic, corrosive, an irritant, a strong sensitizer, flammable, or reactive. Listed under OSHA, 29CFR, part 1910, subpart z. http://www.osha.gov/ Assigned a threshold limit value by American Conference of Governmental Industrial Hygienists (ACGIH) Routes of Entry Inhalation Eye a. gases/vapors Contact Skin Contact/Absorption Ingestion b. particulates Types of Exposure Acute Exposure (brief period of time) Chronic Exposure (months, yrs, decades) III. Exposure Limits Several agencies, each with its own set of standards. Most standards are merely recommendations, only OSHA’s ‘PEL’s have the force of law. Occupational Safety and Health Administration Permissible Exposure Limit PEL: Allowable limit for air contaminant, repeated exposure without adverse health effects. Ceiling C: Exposure limit not to be exceeded at any time during the workday. Short-Term Exposure Limit STEL: 15 minute time weighted average, not to be exceeded Time-Weighted Average TWA: Average airborne exposure in any 8-hour shift of a 40-hour work week, not to be exceeded. Action Level AL: Exposure level at which certain regulations take effect. (analysis, training, medical monitoring, record keeping) Generally ½ the PEL. Exposure Limits--OSHA PEL—Permissible Exposure Limit which may be expressed as A. B. C. TWA—Time weighted Average (8 hr) STEL—Short term exposure limit (15 min) C—Ceiling limit Exposure Limits--ACGIH American Conference of Governmental Industrial Hygienists TLV—Threshold limit value (can be exposed repeatedly at this level) TLV-TWA TLV-STEL TLV-C Exposure Limits--NIOSH National Institute of Occupational Safety and Health REL—Recommended exposure limit (40hr work week) IDLH—Immediately Dangerous to Life and Health Toxicity Toxicity--NIOSH LC50—Lethal Concentration 50 (respiratory) Kills 50% of test animals after a single exposure in a specific time. LCLO—Lethal Lowest Concentration Low concentration to cause a death in human or animal. LD50—Lethal Kills Dose 50 50% by route other than inhalation Toxicity—NIOSH (cont’d) LDLO—Lethal Dose Low TCLO—Toxic Concentration Low Lowest concentration in air to show toxic effects. TDLO—Toxic Toxic Dose Low effects evident by route other than inhalation. IV. Recognizing the Physical and Health Hazards of Chemicals Hazard Warnings Flammable-acetone, ethanol, benzene Corrosive-ammonia, sodium hydroxide, glacial acetic acid, mineral acids Compressed gases-Ar, CO2, NH3 anh, N2, LN2 Poison-chloroform, cyanide salts, phenol, methyl isocyanate, mercury(II)chloride, carbon tetrachloride IV. Recognizing the Physical and Health Hazards of Chemicals… Explosive - perchlorate salts, barium azide, TNT, picric acid/picrate salts Pyrophoric – activated carbon, aluminum borohydride, magnesium powder Water reactive-barium, calcium, lithium, sodium Combustible-phenol, n-propanol, aniline, benzaldehyde IV. Recognizing the Physical and Health Hazards of Chemicals… Carcinogen-Acrylonitrile, asbestos, benzene, carbon tetrachloride, formaldehyde, lead, PCBs, styrene Infectious Substance-bacteria, viruses, parasites Oxidizer-nitric acid, sodium nitrate, silver nitrate, hydrogen peroxide Radioactive C-14, Kr-74, P-32, U-230 National Fire Protection Association (NFPA) chemical hazard diamond: http://www.atsdr.cdc.gov/NFPA/nfpa_label.html Health (Blue) 4 Danger May be fatal on short exposure. Specialized protective equipment required 3 Warning Corrosive or toxic. Avoid skin contact or inhalation 2 Warning 1 Caution 0 May be harmful if inhaled or absorbed May be irritating No unusual hazard Flammability 4 Danger 3 Warning 2 Caution 1 0 (Red) Flammable gas or extremely flammable liquid Flammable liquid flash point below 100° F Combustible liquid flash point of 100° to 200° F Combustible if heated Not combustible 4 Danger 3 Danger 2 Warning 1 Caution 0 Stable Reactivity (Yellow) Explosive material at room temperature May be explosive if shocked, heated under confinement or mixed with water Unstable or may react violently if mixed with water May react if heated or mixed with water but not violently Not reactive when mixed with water Special W Oxy Notice Key (White) Water Reactive Oxidizing Agent Flashpoint vs. Firepoint Flashpoint Lowest temperature at which a flammable liquid gives off sufficient vapor to form an ignitable mixture with air near its surface or within a vessel. Combustion does not continue. Firepoint The lowest temperature at which a liquid produces sufficient vapor to flash near its surface and continues to burn. Usually 10 to 15 ºC higher than the flashpoint. V. Material Safety Data Sheets (MSDS) Specified by OSHA and must contain the following information in 16 sections (but it’s not standard). 1. Chemical Product and Company ID 2. Composition/Information on Ingredients A. CAS # B. Relative percents C. Exposure limits (here or in Section 8) MSDS (cont’d) 3. Hazards Identification A. B. 4. Description of material and overview of hazards Potential adverse effects including routes of entry and carcinogenic properties. First Aid Measures MSDS (cont’d) 5. Fire-Fighting Measures A. B. C. D. 6. Flash point—lowest temperature at which vapors and air form an ignitable mixture Auto-ignition temperature Lower Explosive Limit (LEL) Upper Explosive Limit (UEL) Accidental Release Measures—Spill, leak and response procedures MSDS (Cont’d) 7. 8. 9. 10. Handling and Storage requirements—prevent contact from incompatibles. Exposure Controls/Personal Protection (can be listed in Section 2) Physical and Chemical Properties Stability and Reactivity—provides incompatibilities MSDS (Cont’d) 11. 12. 13. 14. 15. 16. Toxicological Information—usually results of animal testing Ecological Information—what happens in the environment Disposal Considerations* Transport Information Regulatory Information Other Information MSDS (Cont’d) *Section 13 - Disposal Considerations Chemical waste generators must determine whether a discarded chemical is classified as a hazardous waste. US EPA guidelines for the classification determination are listed in 40 CFR Parts 261.3. Additionally, waste generators must consult state and local hazardous waste regulations to ensure complete and accurate classification. RCRA P-Series: None listed. RCRA U-Series: None listed. potassium permanganate VI. First Aid More concerned about (rare) serious incidents rather than the more frequent minor ones (cut finger, minor burn to extremity). Students/Instructors not required to render first aid but knowing some basics can save a life. In the event of an accident: 1. Pause to assess the situation. Consider your own safety. 2. Call (or send) for help. Be specific about the nature and the seriousness of the problem. 3. Monitor situation until help arrives. First Aid Basics a. Acids and bases are corrosive, damaging to tissue. Treat by flushing with large amounts of water. Eyes must be held open. Do not try to neutralize acid with base, reactions are exothermic. b. Organic solvents produce sweet smelling vapors. Should always be used in a hood. If you smell vapors and begin to feel lightheaded, close hood and get some fresh air. First Aid Basics c. Ingestion. Do not induce vomiting. Corrosive materials can do more damage on the way up than on the way down. Give large amounts of water. Solvents can be aspirated. If vomiting occurs keep the person’s head below hips and body on its side to minimize aspiration. d. Fire Large fire unlikely in Chemistry or Biology lab. Fire in a small container can be put out by smothering..Think ahead, have something available. Clothing on fire—SDR, fire blanket, safety shower First Aid Basics d. Fire…(cont) Even moderately large fires can produce significant amounts of noxious and toxic gases. In such a case, leave it to the professionals. e. Electrocution Never touch anyone in contact with live electrical current. Always disconnect power first. VII. Spill, Leak and Disposal Procedures 1. Be prepared 2. Protect yourself 3. Evacuate the immediate area 4. Identify the spilled material 5. Isolate the spill from related hazards 6. Contain the spill 7. Clean up the spill Spills, Leaks……. 8. Dispose of the material 9. Clean yourself up 10. Learn from the experience VIII. Personal Protective Equipment 1. Eye and Face 2. Clothing 3. Gloves 4. Respirators IX. Laboratory Protocol and Techniques The Four G’s of Lab Protocol 1. General 2. Glassware 3. “Get rid of” or Dispose 4. Gear 1. General Drawers/Doors Aisles/Floor Work Surface Cosmetics/Food Smoking Jewelry 2. Glassware Clean/Defect Clutter Disposal Washing Glass Tubing Free 3. Disposal Containers Separate Types Drains Reactive Chemicals Ordinary Trash Contractors 4. Gear Fume Hood Laser UV Sources Compressed Gas Vacuum Dessicator Centrifuge Refrigerator 4….Gear……. X-Ray Generators/Particle Accelerators Extraction/Distillation Cooling Methods X .OSHA Regulations Hazard Communication Standard Laboratory Standard HAZWOPER Standard Blood Borne Pathogens Standard XI. Environmental Protection Agency Clean Air Act-1955 Clean Water Act-1972 Resource Conservation and Recovery Act-1976 Comprehensive Environmental Response, Compensation, and Liability Act-1980 Superfund Amendments and Reauthorization Act1986 XII. General Lab Safety Tips 1. Plan ahead. Consider hazards before performing experiments. 2. Know emergency responses. Locations of extinguishers, eyewash, shower, spill kit, telephone. 3. Know what you are working with. MSDS. 4. Know and follow safety procedures. Goggles, protective equipment, special handling, hoods. …..General Lab Safety Tips….. 5. Report dangerous activities or situations. 6. Store and Handle Hazardous Materials Safely 7. If you don’t know…..ask!