The Sulfur Cycle
advertisement
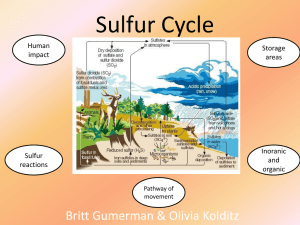
The Sulfur Cycle The sulfur cycle is the collection of processes by which sulfur moves to and from minerals (including the waterways) and living systems. Sulfur In nature: it can be found as the pure element, and as sulfide and sulfate minerals. Commercial uses: fertilizers, gunpowder, matches, insecticides, fungicides, vitamins, proteins and hormones. It is critical in the environment, climate and the health of ecosystems. Its the 10th most abundant element in the universe and 7th most abundant element in our body. Amino acids: Cystein and methionine Biological importance Amino acids: Methionine and Cysteine Therefore important part of proteins, enzymes etc. Vitamins WHERE is sulfur found? The majority of Earth's sulfur is stored: In rocks underground! In sulfur salts at the bottom of the ocean! Sulfur Cycle In ground: most found in rocks, or salt in earth, or as sediment at bottom of ocean Found as S, H2S, SO4-2, (NH4)2SO4 Enter ground: Plants absorb, or left by acid deposition (fog or precipitation) As SO4-2, (NH4)2SO4, and then turn H2S by bacteria, decay, and plant use Stored: Ground, rock, ocean, somewhat in air Sulfur Cycle Sulfur is transferred into biosphere then back into ground, or from ground to atmosphere Microorganisms turn it into H2S (gas) Oxidized in atmosphere to SO2, and then to H2SO4 (an acid) with water contact Mined ores released to atmosphere in factories as H2S and SO2 Volcanoes and hot springs Sulfur Cycle Deposited next in water Through precipitation, dry deposition, leaching Rainfall= deposited 73E12 grams sulfur in 1960 SO4-2 leaches from soil into ocean as sediment H2SO4 falls into ocean Dimethyl Sulfide, carbonyl sulfide (biogenic gases), released by plankton returns back into atmosphere (turns into SO2) Either re-evaporated, left as sediment for long time, or deposited on land 20E12 grams of sulfur a year deposited on land by sea When back on land, cycle repeats The Atmospheric Portion Volcanic eruptions, breakdown of organic matter in swamps and tidal flats, and the evaporation of water, especially seawater, release sulfur directly into the atmosphere. Sulfur eventually settles to earth or comes down with rainfall. Driving Force Driven by: constant addition of sulfur to environment by earths interior (geosphere) Human disturbance, addition of sulfur to atmosphere, (also dug up from environment) Natural processes (incl. Biological, hydrological, due to sun energy) Plant uptake, microbes (Desulfovibrio sp. or Desulfotomaculum sp.) Biochemicaltransformations Physical Weathering release of sulfides (HS-) or sulfates (SO4-3) from minerals Biological transformations: Aerobic sulfur-oxidizing bacteria ; sulfides are converted to sulfate (SO4-2) sulfate is assimilated by plants and microbes Anaerobic sulfate-reducing bacteria; sulfate converted to sulfides Aerobic or anaerobic Mineralization of organic S, release as either HS- or SO4-2 Human Activities The burning of fossil fuels and processing of metals releases huge quantities of sulfur into the atmosphere. Human activities are responsible for one-third of all sulfur emissions and 90% of all sulfur dioxide emissions. Sulfur dioxide emissions lead to acid rain as sulfur dioxide reacts with water to form H2SO4 and sulfur trioxide reacts with water to form H2SO4. Human Effect When mine ores, sulfur/sulfides released into soil Combustion of fossil fuels Release of SO2, causes acid rain, increases amount already present 28% of sulfur in rivers from pollution, mining, erosion, etc. Help move cycle but also upset balance- too much S means acid rain Hydrodesulphurization (refine hydrocarbons)- surplus of S Conclusion Sulfur Cycle is important to biological and natural processes although human’s role impacts nature in a negative way