Closed Loops - American Water Treatment
advertisement

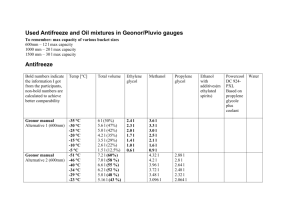
Closed Loops Chilled Water, Hot Water, Heat Pump and Glycol Loops Basic Terminology What are we talking about? Close System – Any water recirculated system that is completely sealed and does not regularly or by design take on makeup Fluid Cooler – A type of cooling tower wherein the primary heat exchanger is located inside the tower. Tower or spray water cascades over the heat exchanger cooling the loop water inside. Heat Pump Loop – Most common type of closed loop system that is used with a fluid cooler. Designed to provide a more highly controlled air temperature by being both heated and cooled within a tight temperature range. Chiller – A piece of HVAC equipment that is used to create chilled water. Commonly part of a cooling tower/condenser system. Glycol – A heat transfer fluid that can decrease the freezing point of a water solution. The two most common types are ethylene and propylene glycol Freeze Point – The temperature at which a water solution begins to form ice crystals Burst Point – The temperature at which a water solution becomes solid and expands Anaerobic Bacteria – Bacteria, typically slime forming, that do not require oxygen to grow. Why Treat a Closed Loop? • • • • Water and Metal don’t mix! • The metal surfaces of your closed loop system are meant to last decades and they can if they are properly protected. A chilled water system line can last 40 – 50 years with proper treatment, but they can fail in as little as a couple years without treatment. Hot water system lines have been known to fail in the first year without treatment. Hot Water Systems can become scaled. • Calcium deposits can form on heating surfaces in areas with high hardness. Proper treatment can prevent any scale formation. Anaerobic bacteria can destroy a closed loop system. • Even though the system is not exposed to sunlight or air, bacterial can still grow in it. Glycols are more corrosive than water by itself. • The corrosion rate of carbon steels and copper alloys in a 30% glycol solution is 1015 times greater than in plain water. What to watch for in a Loop Closed loop systems should be the easiest to treat and they are, but when things go wrong they are also difficult to correct. Dirt and Particulate can damage pump impellers and seals Excessive treatment levels can raise the pH of the loop water over a safe level, yes a high pH can be as bad as a low pH Normal Loop pH should be between 9.0 – 11.0, unless there is aluminum in the system, where it must be kept in the 8.0 – 8.8 range A low pH is bad as everyone knows. Acidic pH’s, those less than 8, can cause accelerated corrosion. If the pH of a glycol solution is acidic it can cause the glycol to breakdown which will push the pH even lower. Bacteria in the closed loop is the hardest problem to correct. Anaerobic bacteria excrete acidic wastes which breakdown the system metals, the inhibitors and glycols. The slimes formed by many types of anaerobes can insult the heat exchangers and reduce flow. Iron oxides and copper oxides, aka rusts, have no place to go in a closed system, therefore they must be kept at a minimum. If not they can clog small diameter ports, contribute to the particulate problem mentioned above and most importantly feed “iron-related” bacteria. Propylene and Ethylene Glycols, Methanol / Ethanol, and Antifreezes Not all antifreeze agents are the same. There are many differences but these are probably the most important. Ethylene Glycol is toxic and is considered a hazardous material. Propylene Glycol has a low toxicity and is actually used in cosmetics and cheap ice cream. Propylene Glycol is more viscous so it has more impact on head pressure . Propylene Glycol has better heat transfer characteristics than Ethylene Glycol. It takes a little more PG than EG to get the same freeze point. Methanol can be used in place of glycols where the pump-ability is a major issue. Methanol is less viscous than water so it will not cause head loss. Methanol and Ethanol are highly flammable in their concentrated states Glycols breakdown, either due to age, bacterial metabolization, or low pH. The breakdown can happened very quickly and in most cases its not something you can fix. Start Off Right Clean Loops are Happy Loops The key to successful closed loop treatment is starting off with a clean system New closed loop piping should always be flushed with an detergent or alkaline cleaner, not just STP, prior to commissioning. Older systems with rusty or smelly water can be alkaline flushed, but this should be followed by a biodispersent flush to remove bacterial slimes. Never put glycol in a system that is not absolutely clean. Glycol can act as a solvent and bring more particulate and discoloration to the water. A little amber discoloration is normal and not an indication of corrosion or other problems, but any foul smell is not a good sign.