ppt
advertisement

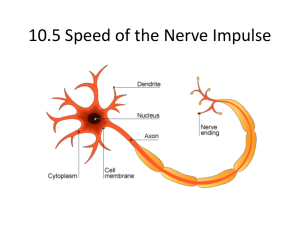
NEW NEUROPHYSIOLOGY LABORTORY COURSE TO START AT UK SPRING 2013 R.L. Cooper , J. Titlow, Dept of Biology, Univ. of Kentucky; Z. R. Majeed, Dept .of Biology, Univ. of Kentucky & Dept .of Biology, College of Sci, Univ. of Salahaddin, Erbil, Iraq Overview of course: Extracellular experiments: Short term facilitation at the NMJ : Joint proprioceptive organ in a walking leg Anatomical 1.0 Fatt and Katz in 1951 showed that acetylcholine was the transmitter that depolarized the frog motor endplate and that voltages would summate. The undergraduate students are to be exposed to the marvels of Neuroscience and to experience the future of the field by learning "Hands-On" neurophysiology, analysis, and neuropharmacology in a laboratory based atmosphere. Relative amplitude Introduction 1.2 Both the frog and crustacean NMJs were the models of choice for investigating the frontiers of facilitation for many years. This was most likely due to the robust nature of these experimental preparations. Goal of course: 0.8 0.6 0.4 0.2 0.0 -0.2 The goal is to train future students in various science disciplines to the integrative nature of science so that they can better prepare themselves with the appropriate training during the remaining years of undergraduate schooling and help to direct their efforts and thus competitiveness towards particular graduate programs. By the end of this course, one should: 1. Have a conceptual understanding of the information processing in the nervous system. 2. Understand the molecular mechanisms that enable signal transmission in the nervous system in terms of receptor potentials, synaptic potentials and action potentials. 3. Know the cellular specializations and the molecular machinery involved in neuron-neuron communication at the state of the art level. 4. Develop a basic knowledge of sensory processing. 5. Be able to understand and critically analyze research papers in the field of Neuroscience. 6. Be able to develop new ideas and suggest future research directions in the field of Neuroscience. A typical intracellular response of STF in the opener muscle of the crayfish walking leg is shown here: Recordings can be made with extracelluar electrodes. The signals from the cut nerve endings will be small in amplitude. To be able to visualize the signals, one needs to amplify the electrical response with either a preamplifier and/or the gain on the oscilloscope. The preamplifier can be set at a gain of 1,000 times. If audio amplifiers are available, they can be used to help hear the signals. The suction electrodes consists of a syringe with a hypodermic needle attached, a replaceable tip made of polyethylene tubing, and two insulated silver wires. Methylene blue of motor nerve terminals for the superficial flexor muscles of the crayfish Tonic and phasic NMJs : Crayfish muscle Anatomy Outline of course: Week 3. Measure facilitation and depression in tonic and phasic neuromuscular junctions in crayfish abdomen muscles. Week 4. Learn to record from proprioceptors (extracellular) in the crab leg and relate to joint positions. Week 5. Learn to record from tension receptors in the crab leg related to muscle length and force. Week 6. Learn how to forward fill neurons from the crab leg proprioceptors (CoCl2, 4-Di-2 ASP) as well as stain with methylene blue. Intracellular experiments: Measuring membrane potentials in crayfish muscle fibers: The students will learn how to properly record the potential across a membrane, with glass electrodes, in the DEL1 and DEL2 muscles in a crayfish. The students furthered their investigation of membrane potentials by determining the effects of increased extracellular K+ levels. Using several solutions of increasing K+ levels, the cells were covered and allowed to soak for 5 min. The resting membrane potential was then recorded again. The students will graph their values for the resting potential against the log potassium concentration and compare these values to the theoretical values determined by using the Nernst equation. EPSP mEPSP Leech ganglion preparation : Leech Ganglion- identified neuron cell body Changing Extracellular K+ -20 Rp (mV) -30 -40 -50 •A square impulse will be applied to the Rz cell. •The initial hyperpolarization and final depolarization indicates the capacitance artifact. •The artifact hyperpolarization and depolarization also indicate the length of the square impulse applied to the Rz cell. •Length of square impulse stimulus was approximately 300 ms. -60 -70 Week 8. Mapping skin receptive fields on the leech while recording from neurons. Dye fills. -80 -90 0 10 20 30 40 50 60 70 [K+]o Experimental Theoretical Week 9. Learn how to remove and culture leech neurons for forming synapses in culture. Week 10. Vision: crayfish & fruit fly eyes and caudal photo receptor in crayfish. Week 11. Quantal analysis of synaptic transmission: Crayfish NMJ record quantal responses. Week 12. Plot I V curves and use Ohms law to determine Rm: Crayfish skeletal muscle. Pharmacology of glutamate receptors Week 13. Student presentations. Intracellular approach Quantal Analysis -10 Week 7. Learn how to dissect the leech ventral nerve cord and obtain intracellular recordings from identified neurons. Current injections and threshold measures. Potentially two intracellular electrodes and record in situ synaptic connections. Investigate the ionic currents making up the action potentials. Muscle tension and tension receptors from a crab leg Lucifer yellow staining of a Rz and P cell in the ganglion by intracellular pressure injection. Week 1. Learn about equipment (extracellular & intracellular amps, microscopes, electrode puller). Solutions and laboratory tools. Animal care. Lab notebooks & reports. Week 2. Measure membrane potentials in crayfish abdomen muscles and plot Rp vs [K]o graphs. Also learn how to stimulate motor nerves and record EPSPs/IPSPs. One can see individual axons to correlate to the different size extracellular spikes that are recorded. Also a goal can be to correlate innervation patterns on the muscle for the different types of terminals and the physiological responses. These are traces of individual spontaneous events that can be recorded and used for measures. Students will compared these types of traces to examine if subsets of events are present. The purpose is to look for quantal events that fall into distinct groupings. Measuring synaptic potentials in crayfish muscle fibers: Record excitatory and inhibitory junctional potentials (EJP's and IJP's) will be a goal fro the students. Recording action potentials extracellularly from the superficial branch of the third root using a fine-tipped suction electrode applied to the side of the nerve, and match different sized spikes in the nerve with junctional potentials in the muscle fibers is another goal. By penetrating several muscle fibers, one can see that not all fibers are innervated by all the efferent neurons, and that the same neuron may elicit different-sized junctional potentials in different fibers. Make a map to show distribution of the EJP's and IJP's in the muscle. We also induced reflex firing of some motor neurons by stroking the sides of the abdomen with a fine brush. Leech ganglion shown below the patch of skin. One can map receptive fields on the skin while recording intracellularly from different sensory neurons. Synaptic field potentials can be measured with focal macropatch electrodes to assess presynaptic vesicular events. The synaptic potentials can be obtained using the loose patch technique by lightly placing a 10-20 m firepolished glass electrode directly over various regions on a muscle fiber. The evoked field excitatory postsynaptic potentials (fEPSPs) and field miniature excitatory postsynaptic potentials (fmEPSPs) can be recorded and analyzed to determine the mean quantal content (m). Direct counts of the number of evoked quantal events and failures in evoked release are to be used as an index of altering synaptic function. In addition to the direct quantal counts, the area of the evoked and spontaneous events will be measured over time in each preparation for comparison. The area of the evoked and spontaneous events will be determined by the Simpson's method. One can record extracellular spikes from the tension nerve while monitoring the development of force for direct correlations Computational Swimmy is a virtual neural circuit simulator designed by Frank Krasne and his colleagues at UCLA. The free software and supporting materials are part of an inquiry-based project that covers basic neurophysiological principles. Students learn how central pattern generators function and correlate neural circuit physiology to animal behavior. Cells in the circuit are silenced or activated by simulating current injections. Then based on activity at the cellular level (below) and the behavioral level (above) students derive the circuit’s connectivity.