Aren*t we just a bunch of wasters?
advertisement
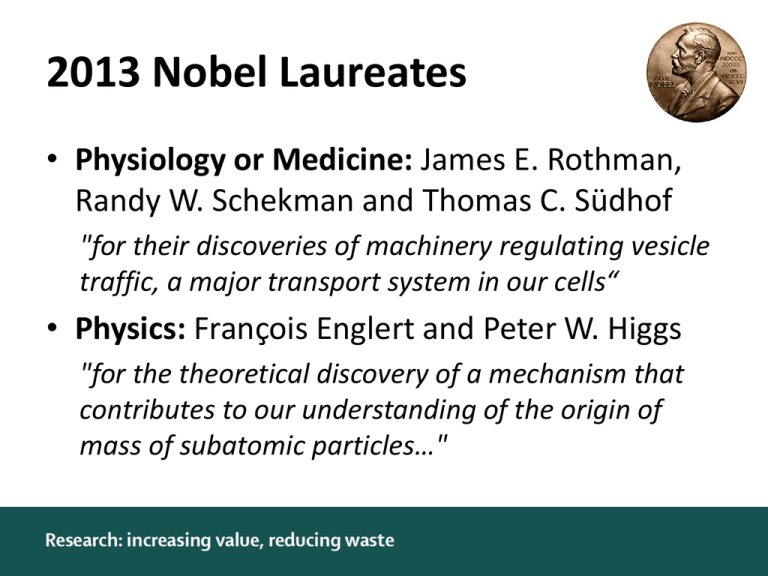
2013 Nobel Laureates • Physiology or Medicine: James E. Rothman, Randy W. Schekman and Thomas C. Südhof "for their discoveries of machinery regulating vesicle traffic, a major transport system in our cells“ • Physics: François Englert and Peter W. Higgs "for the theoretical discovery of a mechanism that contributes to our understanding of the origin of mass of subatomic particles…" Randy W. Schekman Peter W. Higgs • sdf Life sciences research in 2010 US$ 240,000,000,000 85% wasted Lancet 2013;382:1286-307 & 2009;374:86–9 Avoidable waste or inefficiency in biomedical research Are research decisions relevant to users of research? Appropriate research design, methods, and analysis? Efficient regulation and management? Fully accessible research information? Unbiased and usable research reports? Research waste Lancet 2014;383:101–4 The 42 “Wasters”… A Metin Gülmezoglu, Andrew Vickers, An-Wen Chan, Ben Djulbegovic, David Moher, David W Howells, Davina Ghersi, Douglas G Altman, Elaine Beller, Elina Hemminki, Elizabeth Wager, Fujian Song, H Bart van der Worp, Harlan M Krumholz, Iain Chalmers, Ian Roberts, Isabelle Boutron, Janet Wisely, John P A Ioannidis, Jonathan Grant, Jonathan Kagan, Julian Savulescu, Kay Dickersin, Kenneth F Schulz, Malcolm R Macleod, Mark A Hlatky, Michael B Bracken, Mike Clarke, Muin J Khoury, Patrick Bossuyt, Paul Glasziou, Peter C Gøtzsche, Robert S Phillips, Robert Tibshirani, Rustam Al-Shahi Salman, Sander Greenland, Sandy Oliver, Silvio Garattini, Steven Julious, Susan Michie, Tom Jefferson, Ulrich Dirnagl 1. Setting research priorities Lancet 2014;383:156–65 1. Setting research priorities The inefficiency of basic science research 1 intervention used widely 5 resulted in licensed clinical interventions by 2003 101 claimed that new discoveries had clear clinical potential >25,000 reports in 6 basic science journals 1979-83 Am J Med 2003;114(6):477-84 1. Setting research priorities Thomas Edison "Young man, why would I feel like a failure? And why would I ever give up? I now know definitively over 2,000 ways that an electric light bulb will not work. Success is almost in my grasp.“ 1. Setting research priorities Best basic:applied research funding ratio? Lancet 2014;383:156–65 1. Setting research priorities Whose priorities? Lancet 2014;383:156–65 1. Setting research priorities Involve stakeholders Lancet Neurol 2012;11:209 1. Setting research priorities Set research in the context of systematic reviews Lancet 2014;383:156–65 1. Setting research priorities What systematic reviews could have shown… tPA in animal stroke models prone:supine SIDS:controls Lancet 2014;383:156–65 1. Recommendations • • • • More research on research should be done to identify factors associated with successful replication of basic research and translation to application in health care, and how to achieve the most productive ratio of basic to applied research Research funders should make information available about how they decide what research to support, and fund investigations of the effects of initiatives to engage potential users of research in research prioritisation Research funders and regulators should demand that proposals for additional primary research are justified by systematic reviews showing what is already known, and increase funding for the required syntheses of existing evidence Research funders and research regulators should strengthen and develop sources of information about research that is in progress, ensure that they are used by researchers, insist on publication of protocols at study inception, and encourage collaboration to reduce waste Lancet 2014;383:156–65 2. Design, conduct and analysis Incongruent statistical findings in publications in 2001 (rounding, transcription, or type-setting errors) 25% 38% 62% 75% BMC Med Res Methodology 2004;4:13 2. Design, conduct and analysis Failure to replicate published pre-clinical academic results 11% 36% 64% 89% Lancet 2014;383:166–75 2. Design, conduct and analysis High effect-to-bias ratio In vivo studies Lancet 2014;383:166–75 2. Recommendations • Make publicly available the full protocols, analysis plans or sequence of analytical choices, and raw data for all designed and undertaken biomedical research • Maximise the effect-to-bias ratio in research through defensible design and conduct standards, a well trained methodological research workforce, continuing professional development, and involvement of nonconflicted stakeholders • Reward (with funding, and academic or other recognition) reproducibility practices and reproducible research, and enable an efficient culture for replication of research Lancet 2014;383:166–75 3. Regulation and management “…the clinician who is convinced that a certain treatment works will almost never find an ethicist in his path, whereas his colleague who wonders and doubts and wants to learn will stumble over piles of them” Lancet 1990;336:846–7 3. Regulation and management Delays and inconsistency in ethics and governance Lancet 2014;383:176–85 3. Regulation and management Is regulation proportionate, when the public approves? 23% 28% 72% UK National Cancer Registry including postcode, name and address, and sending a letter inviting them to a research study 77% Finland national biobank of existing diagnostic and research samples Lancet 2014;383:176–85 3. Regulation and management RCTs recruited pre-specified sample size 31% 45% 55% 69% 114 RCTs funded by MRC or HTA in the UK in 1994-2003 73 RCTs funded by MRC or HTA in the UK in 2002-2008 HTA 2007;11:ix–105 & Trials 2013;14:166 3. Regulation and management Better recruitment after UK clinical research networks Lancet 2014;383:176–85 3. Recommendations • People regulating research should use their influence to reduce other causes of waste and inefficiency in research • Regulators and policy makers should work with researchers, patients, and health professionals to streamline and harmonise the laws, regulations, guidelines, and processes that govern whether and how research can be done, and ensure that they are proportionate to the plausible risks associated with the research • Researchers and research managers should increase the efficiency of recruitment, retention, data monitoring, and data sharing in research through the use of research designs known to reduce inefficiencies, and do additional research to learn how efficiency can be increased • Everyone, particularly individuals responsible for health-care systems, can help to improve the efficiency of clinical research by promoting integration of research in everyday clinical practice Lancet 2014;383:176–85 4. Accessible reporting Proportion of funded/completed research that is reported 100% 1980 - 1996 80% 1997 - 2005 60% 40% 20% UK US Sw A itz Ge erl. rm an y Sp a Ne i n th er l. U Au K st ra lia Fr an D e ce nm ar Ca k na Au da st ra lia Sp ai n US A 0% HTA 2010;14(8):iii, ix-xi, 1-193 4. Accessible reporting Reporting is selective Lancet 2014;383:257–66 4. Accessible reporting Associations with reporting Oseltamivir Rosiglitazone Rofecoxib/celecoxib etc Lancet 2014;383:257–66 4. Accessible reporting Language bias Chinese Biomedical Literature database Chinese Medical Current Content China National Knowledge Infrastructure Chinese Scientific Journals database Chinese Medicine Premier 2,500 biomedical journals, <6% indexed in Medline Health Info Libr J 2008;25:55–61 4. Accessible reporting Lancet 2014;383:257–66 4. Recommendations • Institutions and funders should adopt performance metrics that recognise full dissemination of research and reuse of original datasets by external researchers • Investigators, funders, sponsors, regulators, research ethics committees, and journals should systematically develop and adopt standards for the content of study protocols and full study reports, and for data sharing practices • Funders, sponsors, regulators, research ethics committees, journals, and legislators should endorse and enforce study registration policies, wide availability of full study information, and sharing of participant-level data for all health research Lancet 2014;383:257–66 5. Complete & usable reporting Lancet 2014;383:267–76 5. Complete & usable reporting Lancet 2014;383:267–76 5. Complete & usable reporting Lancet 2014;383:267–76 5. Recommendations • Funders and research institutions must shift research regulations and rewards to align with better and more complete reporting • Research funders should take responsibility for reporting infrastructure that supports good reporting and archiving • Funders, institutions, and publishers should improve the capability and capacity of authors and reviewers in high-quality and complete reporting Lancet 2014;383:267–76 Avoidable waste or inefficiency in biomedical research Are research decisions relevant to users of research? Appropriate research design, methods, and analysis? Efficient regulation and management? Fully accessible research information? Unbiased and usable research reports? Research waste Lancet 2014;383:101–4 How can, and will, we change? Implementation Science 2011;6:42