presentation slides
advertisement

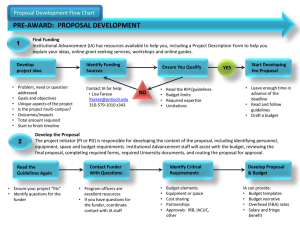
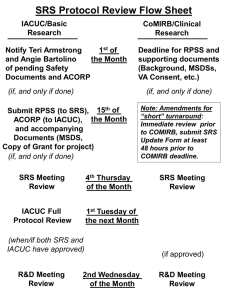
Integrated Online Research Compliance System (iORC) Current Situation Research involving the use of Hazardous materials & equipment Human subjects / Human biological materials Animals Seek clearance from compliance offices IBC (OSHE) IACUC IRB Objectives of the System Harmonise the compliance process One-stop platform for researcher Enhance communication between compliance offices Key Features of System Real time dashboard view Routing of application for review Email notification for action required IACUC application For new application and subsequent amendments (Forms will not be available on IACUC website) Not for amendment of existing protocols (Amendment forms remain on IACUC website) IACUC application – Major changes • Procedure B - combined Procedure B, Procedure D and part of OSHE RA forms. (No Procedure D form) • ‘Actual’ amendment of an approved protocol. (No amendment forms. A checklist for amendment) • Change in format of forms. (Questions asked in form generally remain the same) Why change to iORC?? Legal & safety compliance Reduce administrative burden All submissions on a single portal- new forms amendments, regulatory applications can be uploaded Communication source • • Shared form- OSHE & IACUC form Comments by reviewer/PI/HOD Are there any grounds for a confidentiality breach? All submissions will be confidential and not accessible by unauthorized personnel OSHE - Current OPRAS - Limitations 1. Does not allow Co-PI to be listed and have access to the risk assessment submissions 2. PIs are not allowed to make changes once submitted 3. Communication is via email hence need to document all communications separately 4. PI need to provide same information to OSHE & IACUC on animal work Current OPRAS - Limitations 5. Does not capture training records automatically 6. Grant release forms & Diving research form was not included 7. Amendment is not possible using the system- hard copy manual submission 8. IACUC form is not included Benefits of iORC • RA (OSHE) submission and IACUC applications on the same portal • Shared animal procedure form “Procedure B”– OSHE & IACUC • Allow other project members to draft a risk assessment (or IACUC protocol) on PI’s behalf • HOD would be able to approve online • Forms ensure all required fields are properly filled up upon submission, thus reducing processing time Benefits of iORC • Allow PI to submit the following forms online • • • Grant release form- Certified PIs Field research form- (both certified & non-certified PIs Diving research form Amendment form • Online approval letter sent to respective email accounts • hard copy approvals not necessary Benefits of iORC • Attachments feature – Allows SOPs, inventory lists, Regulatory applications, SDS to be submitted with the RA. • PIs able to edit the content after submission & track the process of each submission and clarify with the processing officer directly • “Edit Function” – Templates of original risk assessment can be edited for amendment submission OSHE Forms Project Details Form (This is a MANDATORY form.) Lab Certification form if you are under Lab Certification Scheme (currently hard copy submission) Field Research Assessment (currently hard copy submission) Diving form if you are conducting Diving Research (currently manual hard copy submission) Laboratory based project risk assessment form • • • • • • • • • • Biological Chemicals Radiation Animals Procedure B (Shared form for OSHE & IACUC) Electrical Mechanical Noise Ergonomic Other Hazards Risk Summary (This is a MANDATORY form.) Amendment (currently manual copy submission) OSHE Review Process FAQs for OSHE forms under iORC How to get started…. • Select new application to get to following page • OSHE forms can be found under safety and health clearance What purpose can iORC be used for? • Applying for release of new grants for research PIs –certified under the lab certification –Not certified under lab certification • Approval for new animal protocols • Approval for new field research work projects • Approval for new diving research work • To make an amendment for an approved application submitted through iORC What are the things iORC cannot be used for? • To make amendments for earlier applications submitted – through OPRAS – by hard copy submissions • To make amendments to earlier approved field research projects Teaching and student protocols need not be submitted through iORC for OSHE review Are there any mandatory OSHE forms? • Project details and risk summary are mandatory forms • MUST BE FILLED for all types of applications Which forms should be filled for grant release if the PI is lab certified and there is no animal work? Which OSHE form should be filled If the PI is doing field research work? Even if the PI is certified under lab certification select this form When should animal procedure B form be submitted? • For IACUC – when animals are subjected to nonsurgical procedure, including the use of hazardous materials. • For OSHE – when hazardous material (biological, chemical, radiological etc) are used on animals • Therefore this form is common for both IACUC and OSHE submissions when hazardous material is used • Will be reviewed by both offices If procedure B is common need it be selected twice? • No • If procedure B is selected under animal work forms it will automatically selected under OSHE forms and vice-versa If PI is certified, which other forms should be selected if procedure B is involved? Select the relevant main form under Animal Use forms Select the Lab Certification Form and the other two mandatory forms If PI is not certified, which other forms should be selected if procedure B is involved? Select the relevant main form under Animal Use forms Select other relevant sections under Step 3 and the other two mandatory forms Where should the procedure B be filled and is it necessary to fill it twice? • Procedure B should be selected under animal forms • It is not necessary to fill it twice Is it possible to submit only IACUC forms for research work without any OSHE forms when procedure B is selected? NO! Is it possible to submit OSHE forms without IACUC forms when animal procedure B is involved? NO! What if a certified PI complete only OSHE forms and forget to select animal work forms? When filling the lab certification form there will be a prompt to ask whether animal work is involved How can certified PI inform OSHE of use of regulated biological agents and GM work Is it possible to attach documents when using iORC? • Yes, it is possible to attach documents in places where the option is provided by the system • E.g. when submitting GMAC form Do we need HOD to endorse the form? • Once PI submit the online form, HOD will be notified and need to support the application before processing. Do PIs receive the hard copy approval once the risk assessment is approved by OSHE? • No, They will receive the approval in PDF version in email, it will also be automatically saved in the system for future reference. Thank You! Questions??