Pre-Award Basics - Office of Research Administration – Department
advertisement
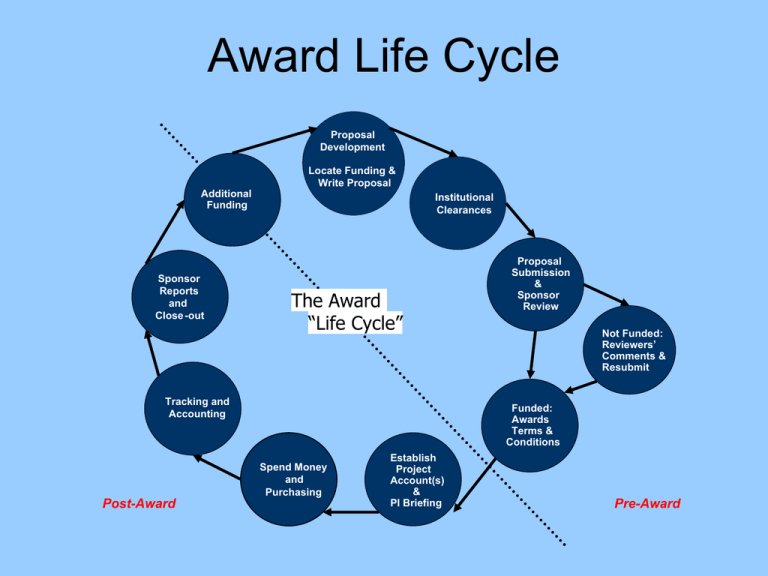
Award Life Cycle Proposal Development Locate Funding & Write Proposal Additional Funding Sponsor Reports and Close -out Institutional Clearances The Award “Life Cycle” Tracking and Accounting Not Funded: Reviewers’ Comments & Resubmit Funded: Awards Terms & Conditions Spend Money and Purchasing Post-Award Proposal Submission & Sponsor Review Establish Project Account(s) & PI Briefing Pre-Award Pre-Award Basics Grants • A grant is a type of financial assistance awarded to an organization for the conduct of research or other program as specified in an approved proposal. For an award to be considered a grant, it will contain the following elements: – Statement of work allows the PI significant freedom to change the emphasis within the general area of work as the project progresses – Deliverables are minimal, consisting typically of reports – Separate accounting procedures are required – Grants most often use the cost-reimbursement method of payment Contracts • A contract is an agreement to acquire services that primarily benefit the sponsor. For an award to be considered a contract, it normally must contain all of the following elements: – Detailed financial and legal requirements included with a specific statement of work – A specific set of deliverable and/or reports to the sponsor – Separate accounting procedures – Legally binding contract clauses Contracts • Contracts, by their nature, are restrictive, meaning there is little flexibility on the part of the academic department to rebudget funds without prior sponsor approval. Invoicing on contracts can be more complex, especially if tied in with milestones or tasks that must be completed by the PI before an invoice can be submitted and/or paid. Clinical Trials • The controlled, clinical testing in human subjects of investigational new drugs, devices, treatments, or diagnostics, to assess their safety, efficacy, benefits, costs, adverse reactions, and/or outcomes. Such studies may be conducted under an industry-developed protocol or an investigator-developed protocol. UCLA Central Pre-Award Offices • Non-Profit Grants – OCGA Analyst • Non-Profit Contracts – OCGA Officer • Industry Sponsored Research Contracts (not including Clinical Trials) – Office of Intellectual Property & Industry Sponsored Research (OIP-ISR) • Industry Sponsored Clinical Trials – DGSOM Clinical Trials Administration Office (CTAO) Project Period • The Project Period is the total time for which support of a project has been programmatically approved. A project period may consist of one or more budget periods. Budget Period • A budget period is the interval of time, usually 12 months, into which the project period is divided for budgetary and funding purposes. • Example: An award with a project period of July 1, 2007 – June 30, 2010 would have 3 budget periods: – (1) July 1, 2007 – June 30, 2008 – (2) July 1, 2008 – June 30, 2009 – (3) July 1, 2009 – June 30, 2010 NIH Grant Numbers • Example: 2 P01 HL030568-26A1 – 2 = Type of application – P01 = Activity Code – HL = NIH Institute to which the grant was assigned (National Heart Lung & Blood Institute) – 030568 = Serial # assigned by Center for Scientific Review – 26 = Year of support of the grant – A1 = Other information identifying a Supplement (S1), Resubmission (A1), or a fellowship’s institutional allowance NIH Application Types • Type 1 – New • Type 2 – Renewal (Competing Cont.) • Type 3 – Application for additional (supplemental) support • Type 5 – Non-competing continuation • Type 7 – Change in grantee institution • Type 9 – Change in NIH awarding Institute NIH Activity Codes • Research Grants (R series) • Career Development Awards (K series) • Research Training & Fellowships (T&F series) • Program Projects/Center Grants (P series) • Cooperative Agreements (U series) NIH Definitions of Proposal Types • New (Type 1) – refers to an application not previously proposed. • Resubmission (A1) – Grants.gov term for a grant application resubmitted to NIH after a PI applicant who did not succeed in getting funded revised it based on feedback from the initial peer review. Formerly known as a revision • Revision (S1) – Grants.gov term for money added to a grant to expand its scope or meet needs of a research protocol. Applicants must apply and undergo peer review. Also known as a competing supplement. NIH Definitions of Proposal Types • Renewal, aka Competing Continuation (Type 2) – An application requiring competitive peer review and Institute/Center action to continue beyond the current competitive segment. • Non-Competing Continuation aka Progress Reports (Type 5), – A year of continued support for a funded grant. Progress reports for continued support do not undergo peer review, but are administratively reviewed by the Institute/Center and receive an award based on prior award commitments. Funding Opportunity Announcements (FOA) • Program Announcement (PA) – A formal statement about a new or ongoing extramural activity or mechanism. It may serve as a reminder of continuing interest in a research area, describe modification in an activity or mechanism, and/or invite applications for grant support. • Request for Applications (RFA) – A formal statement that solicits grant or cooperative agreement applications in a well-defined scientific area to accomplish specific program objectives. An RFA indicates the estimated amount of funds set aside for the competition, the estimated number of awards to be made, and the application submission dates. Applications submitted in response to an RFA are usually reviewed by a Scientific Review Group (SRG) specially convened by the awarding component that issued the RFA. Solicited vs. Unsolicited • Solicited (also known as Targeted Research) – Research funded as a result of an Institute set aside dollars for a specific scientific area, Institutes solicit applications using research initiatives (PAs/RFAs for grants, RFPs for contracts). • Unsolicited (also known as Investigator-Initiated Research) – Research funded as a result of an investigator, on his or her own, submitting a research application. – For NIH, PIs submit unsolicited proposals using the NIH’s Parent Announcements. Funding Opportunity Announcements (FOA) • Parent Announcements – Electronic grant applications must be submitted in response to an FOA. For applicants who wish to submit what were formerly termed Investigator-Initiated or Unsolicited applications, NIH has developed Parent Announcements. Responding to such an umbrella Parent FOA ensures that the correct application package is used and enables NIH to receive the application from Grants.gov NIH Standard Due Dates for Competing Applications • Standard Due Dates for Competing Applications – Includes Earliest Start Dates NIH Modular vs. Detailed Budgets • Modular - A type of grant application in which support is requested in specified increments without the need for detailed supporting information related to separate budget categories. • Sample Modular Budget • Usually max of $250,000 direct cost/yr, in increments of $25,000 NIH Cost Sharing/Matching • Definition: The value of third party in-kind contributions and the portion of the costs of a federally assisted project of program not borne by the Federal Government. Matching or cost sharing may be required by law, regulation, or administrative decision of an NIH Institute or Center. Costs used to satisfy matching or cost sharing requirements are subject to the same policies governing allowability as other costs under the approved budget. Mandatory Cost Sharing • Contributions to projects that are required by the sponsor as a condition of eligibility and/or are a review criterion. Mandatory cost sharing must be tracked and reported to sponsors and cost shared effort must be added to the individual’s Effort Report during the effort certification process. Voluntary Committed Cost Sharing • Contributions to the project that are not required by the sponsor but are offered in the proposal (either in the budget or justification). Voluntary committed cost sharing must be tracked and reported to sponsors, and cost shared effort must be added to the individual’s Effort Report during the effort certification process. • Example: Effort without salary listed in the budget. Voluntary Uncommitted Cost Sharing • Incidental or unanticipated contributions to sponsored projects that were not pledged in the proposal, are not an obligation of the award, and do not have to be tracked or reported to the sponsor. This type of cost sharing often takes the form of investigator effort over and above that for which support was sought. Pre-Award Resources •DOM ORA website – Pre-Award section (includes preaward manual, forms & reference material) –Proposal Preparation –Proposal Intake Form –Budget Preparation •OCGA Proposal Preparation –Commonly Needed Information –UCLA Employee Benefit Rates •NIH Forms & Applications –SF424 (R&R) Forms and Instructions (Grants.gov) –PHS 398 Forms and Instructions (Paper Submissions) •NIH Glossary & Acronym List Pre-Award Strategies •Send out a monthly email to all PIs requesting information on upcoming proposal deadlines. •Web search for agencies guidelines, instructions & forms. •Start EARLY! •Meet with your PI in person. Use the Proposal Intake Form to help guide your discussion. •If the agencies does not provide a checklist of items required for submission, use the proposal’s Table of Contents as a checklist. •Read through the sponsor guidelines thoroughly, highlighting important instructions •Ask your colleagues for examples of similar proposals that were successfully funded. •Review all documents provided to you for compliance with agencies guidelines, and format to create consistency within the proposal. •Ask for help if you need it! Pre-Award Strategies •Determine the proposal’s complexity –Have you submitted to this agency before, and are aware of their guidelines/policies? –Subawards, foreign and/or domestic? –Detailed budget vs. Modular budget? –Paper vs. Electronic vs. Both? –Number of Key Personnel? –PI’s habits, e.g. always late/always early, expectations, etc.? –Animals and/or human subjects? –Budget over $500,000 in any given year? –Agency provides only non-fillable forms? –Will the grant be submitted via OCGA or DRA? –Do you have numerous proposals due on, or around, the same deadline?






![NIH 101: Part 1 [.ppt]](http://s2.studylib.net/store/data/005398706_1-cbe361c448786ac362a8e75ad39fc05d-300x300.png)
