Uniform Guidance Update - Office of Research Administration
advertisement

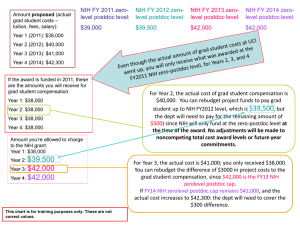
Uniform Guidance Update RESEARCH ADMINISTRATORS TEAM MEETING FEBRUARY 2015 New University Policies Administrative and Clerical Salaries on Federal Sponsored Awards May now charge administrative and clerical salaries to additional types of Federal sponsored awards if specified requirements are met. Requires prior written agency approval, except may be approved through NIH Recipient’s Authorities when applicable. http://policies.iu.edu/policies/categories/research/Sponsored-ProgramsAdministration/admin-clerical-salaries.shtml Reference: 2 CFR Part 200.413(c) New University Policies Computing Devices on Federal Sponsored Awards Costs of computing devices may be allocated as a direct charge to Federal sponsored awards proportionate to the utilization of the devices in the performance of the sponsored award. http://policies.iu.edu/policies/categories/research/Sponsored-ProgramsAdministration/computing-devices.shtml Reference: 2 CFR Part 200.453(c) New University Policies Publication Costs on Federal Sponsored Awards Publication costs may now be direct charged to Federal sponsored awards after the project end date up until 30 days prior to the closeout date. If costs are incurred after the project end date, charge cost to a departmental account then transfer expense to the sponsored program account at least 30 days prior to closeout. http://policies.iu.edu/policies/categories/research/Sponsored-ProgramsAdministration/publication-costs.shtml Reference: 2 CFR Part 200.461(3) New University Policies Visa Charges on Federal Sponsored Awards Costs associated with short-term visas (as opposed to longer-term, immigration visas (Green Cards)) may be direct charged to Federal sponsored awards for personnel working on the performance of the sponsored award. http://policies.iu.edu/policies/categories/research/Sponsored-ProgramsAdministration/visa-charges.shtml Reference: 2 CFR Part 200.463(d) NIH Interim Grant General Conditions Effective December 26, 2014 new NIH grants and funding amendments to existing NIH grants will begin referencing, and are subject to, the Grant General Conditions dated 12/26/14. Based on regulations at 45 CFR Part 75, which implements 2 CFR Part 200. A document released by the NIH on February 5, 2015 contains Interim Grant General Conditions and is intended to highlight changes to NIH general grant conditions resulting from the implementation of the Uniform Guidance. http://grants.nih.gov/grants/policy/NIH%20Interim%20Grant%20General%2 0Conditions.pdf NIH Interim Grant General Conditions Revised Selected Items of Cost Value Added Tax Policy – foreign taxes charged for the purchase of goods and services which recipient is legally required to pay in country are allowable expense under Federal Awards, except in countries which offer an exemption of this tax for research. 45 CFR 75.470(c) Participant Support Costs – for NIH awards, these costs are only allowable when identified in the funding opportunity announcement (FOA). 45 CFR 75.2 Temporary Dependent Care Cost – costs may be direct charged to Federal sponsored awards when certain conditions are met. IU is not currently allowing these costs due to the requirement to be consistent across all funding sources. 45 CFR 75.474 NIH Interim Grant General Conditions Recipient’s Authorities Incur pre-award costs up to 90 days before award beginning date. Initiate a one-time extension of final budget period w/o additional funds. Carryforward Unobligated balances from one budget period to the next. Rebudget among the budget categories. Rebudget between direct and F&A costs. Provide subawards based on fixed amounts when meeting 45 CFR 75.201. Direct charge salaries of administrative and clerical staff if conditions in 45 CFR 75.413 are met. NIH Interim Grant General Conditions Recipient’s Authorities (cont.) Direct charge payments of Incidental activities for which supplemental compensation is allowable under written institutional policy. Rate can’t exceed the IBS. Include charges for Intra-IHE faculty consulting on sponsored agreements that exceed a faulty member’s IBS in unusual cases. Direct charge capital expenditures for general purpose equipment. Prior written approval required for expenditures involving buildings and land. Direct charge capital expenditures for special purpose equipment with a unit cost over $5,000. NIH Interim Grant General Conditions NIH Prior Approval Required Additional no-cost extension greater than 12 months, or late notice of initial no-cost extension. Alteration and renovation. Capital Expenditures involving construction, land, or building acquisition. Carryover of unobligated balances when NoA restricts recipient’s authority. Change in scope. Change in status of the PD/PI or senior/key personnel named in the NoA. Change of recipient organization. NIH Interim Grant General Conditions NIH Prior Approval Required (cont.) Change of recipient organization status. Deviation from award terms and conditions. Foreign component added to a grant to a domestic or foreign organization. Need for additional NIH funding. Pre-award costs when more than 90 days prior to award start date. Rebudgeting funds from trainee costs. Rebudgeting funds between construction and non-construction work. Retention of research grant funds when career development award (CDA) awarded. Questions?