Physical Changes
advertisement

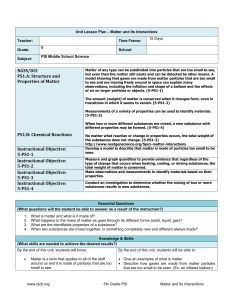
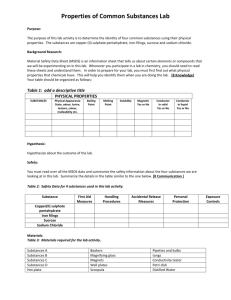
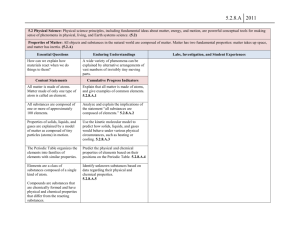
Unit 1 Lesson 3 Physical and Chemical Changes Copyright © Houghton Mifflin Harcourt Publishing Company Discussion: What’s New? Physical or chemical change? What is true about the kinds of matter present before and after a physical change? How is matter different? What substances were formed when the paper burned? What is true about the types of matter present before and after the matter undergoes a chemical change What substances were formed when the paper burned? Would it be possible to change these types of matter back into a sheet of paper? Unit 1 Lesson 3 Physical and Chemical Changes P36 - 38 Change of Appearance Physical Changes • A physical change is a change that affects one or more physical properties of a substance. • The appearance, shape, or size of a substance may be altered during a physical change. • Physical changes, such as changes in state, do not change the chemical identity of a substance. 5) Physical properties of the substance are changed. 7) Yarn was twisted, dyed, and knit to form sweater – it’s still wool. Unit 1 Lesson 3 Physical and Chemical Changes Change of Appearance P38-39 Chemical Changes • A chemical change is the process when one or more substances change into entirely new substances with different properties. • A substance’s identity changes because the chemical makeup changes. • Bonds get rearranged and/or new bonds form. • Influenced by temperature: At higher temperatures, chemical reactions happen more quickly 8) A. Flames B. wood C. Ashes 8) Burning wood is an example of a chemical change in which wood is transformed into new substances, such as ash and smoke P38-39 Smart Activity 9) Raising temp causes particles in matter to move more quickly. Increased motion causes particles to bump into each other which increases the rate of the chemical reaction Let’s go to lab Unit 1 Lesson 3 Physical and Chemical Changes p40 - 41 Look for the Signs How can you tell a chemical change has happened? • There are several signs that a chemical reaction has occurred. • Observing two or more of these signs during a change means you are likely observing a chemical change. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 1 Lesson 3 Physical and Chemical Changes p40 - 41 How can you tell a chemical change has happened? • Odors can be produced during a chemical change. • Fizzing and foaming may mean gases are being produced. • The production of gas is often evidence of a chemical change. •Boiling also can produce gas bubbles, but boiling is a physical change. Unit 1 Lesson 3 Physical and Chemical Changes p40 - 41 How can you tell a chemical change has happened? • A precipitate is a solid formed from liquids. • The formation of a precipitate can indicate a chemical change. • Energy that changes from one form to another can be evidence of a chemical change. • Changes in temperature and color can be signs of a chemical change. Unit 1 Lesson 3 Physical and Chemical Changes P42 - 43 Conservation Is the Law What is the law of conservation of mass? • French chemist Antoine Lavoisier studied chemical changes in which substances appeared to gain or lose mass. • The law of conservation of mass states that in ordinary chemical and physical changes, mass is not created or destroyed. It is only transformed into different substances. Unit 1 Lesson 3 Physical and Chemical Changes P42 - 43 What is the law of conservation of mass? • Physical changes are reversible and follow the law of conservation of mass. • Mass is conserved during chemical changes. The mass of the starting materials is the same as the mass of the end products. Chemical Change