1038950Notes 5.1
advertisement

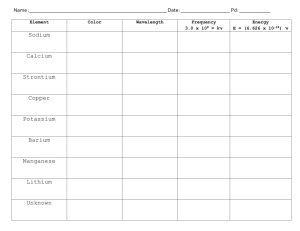
Unanswered Questions • Rutherford’s work on the atom was important but left big questions: – How are electrons arranged around the atom? – Why aren’t electrons pulled into the positive nucleus? – What accounts for the difference in chemical behavior among atoms? Unraveling Chemical Behavior • In the early 1900’s chemists found that certain elements emitted visible light when heated in a flame (now called atomic emission spectra). • They found that this and all of an element’s chemical behavior is related to the arrangement of the electrons in its atoms. Electromagnetic Radiation • A form of energy that exhibits wavelike behavior as it travels through space. • Includes visible light, ultraviolet, infrared, TV, radio, X-ray, microwaves & gamma • All e-m waves travel at the speed of light (c) which is 3.00 X108 m/s. • All e-m waves have a wavelength, frequency and amplitude. • This is the wave model of light. Electromagnetic Spectrum • • • • C=λxv C = speed of light in m/s and λ = wavelength Wavelength is given in meters and frequency is given in hertz or s-1. Wavelength and frequency have an inverse relationship (one goes up the other goes down). Greater frequency = greater energy Try It 1 A helium-neon laser emits light with a wavelength of 633 nm. What is the frequency of this light? Try It 2 What is the wavelength of X rays having a frequency of 4.80 x 1017 Hz? Need for a new model • The wave model of light cannot explain why heated objects emit only certain frequencies (colors) of light at a given temperature • Or why some metals emit electrons when colored light of a specific frequency shines on them. Planck’s Work • Found that heated metals emit specific colors (frequencies) of light (flame tests). • As objects get hotter and possess more energy, the metal emits different colors (frequencies) of light. • Found matter can only gain or lose energy in small, specific amounts (a quantum) Quantum • The minimum amount of energy that can be gained or lost by an atom at a time. • Equantum = hv • Since C = λ x v, then E = hc / λ • h = Planck’s constant: 6.626 x 10-34 Joules.seconds or J.s More quantum ideas • According to Planck’s theory, matter can have only certain amounts of energy— quantities of energy between these values do not exist. • So this was a big deal at the time! Try It 3 Calculate the energy of a gamma ray photon whose frequency is 5.02 x 1020 Hz. = (6.626 x 10-34 J.s) (5.02 x 1020 Hz) = 3.33 x10-13 J Photoelectric Effect Increase light’s intensity and more electrons are ejected. Increase light’s frequency and ejected electrons move faster. Photoelectric Effect • Each metal has a minimum frequency necessary for this to happen. • Varying intensity and frequency changes the number of electrons ejected & their speed. • Led to development of photoelectric cells used in street lights, solar calculators, etc. Einstein’s Proposal • The wave model couldn’t explain the photoelectric effect. • So in 1905 Einstein proposed the “particle nature” of light. • Says that light is both a wave and particle • Light is really a stream of tiny particles or bundles of energy called photons. Photons • Bundles of energy that make up all EM waves like light. • Have no mass but each photon carries a quantum of energy so Equantum = Ephoton = hv Atomic Emission Spectra • If a gas is heated, it absorbs energy which can cause some electrons to be “excited” or bumped up to the next energy level. • These excited electrons are unstable, so emit or lose the energy and drop back to their original energy level. • The emitted energy is seen as different colors of light. • The process continues over & over as long as energy is put into the gas. • Each element has a unique emission spectra that consists of certain frequencies or colors. • Elements can be identified by their spectra. Try It 4 • An FM radio station broadcasts at a frequency of 98.5 MHz. What is the wavelength of the station’s broadcast signal? 98.5 MHz x 106 Hz / 1 MHz = 98.5 x 106 Hz = (3.00 x 108 m/s) / 98.5 x 106 Hz =3.05 meters Try It 5 Calculate the energy of a photon of ultraviolet light that has a wavelength of 49.0 nm. E = (6.626 x 10-34 J.s) (3.00 x 108 m/s) / (49.0 x10-9 m) E = 4.06 X 10-18 J