Document
advertisement

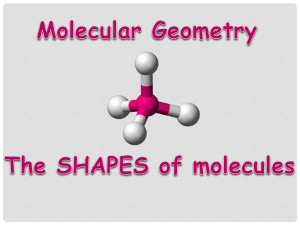
Using Virtual 3-D Technology to Improve Learners’ Spatial and Science Skills: Teaching VSEPR Theory in Second Life® A STUDY Dr. Wendy L. Keeney-Kennicutt Presidential Professor for Teaching Excellence, Piper Professor of 2010 Associate Director, First Year Chemistry Program, TAMU k-keeney@tamu.edu Ms. Zahira Merchant Educational Psychology, TAMU zhmerchant@neo.tamu.edu 1 Premise to investigate if Second Life can enhance – learning of a chemistry concept: 3-D shapes of molecules (VSEPR* theory) – Spatial ability – translating 3-D images into 2-D pictures and back again * Valence Shell Electron Pair Repulsion Theory 2 My Second Life Space on 12th Man Island (maps.secondlife.com/secondlife/12th%20Man/215/237/26) 3 Classroom Use Classroom Area – for reviews and office hours Clicker System Movie Screen Streaming Desktop Blackboard Notice Board Quiz System Seats that allow students to raise their hands 4 TAMU Islands TAM Health Science Center (4 Islands) Communication Island - coming Dr. K’s Chemistry Corner AgriCulture Aggie Orientation Ecosystem TAMUGalveston (2 Islands) TAMU Faculty are using SL to do Meetings Simulations HSC Nursing* Ag Crisis Comm* Ecosystems* Student Talks* Guest speakers Tech Writing Office hours & Reviews Learning modules Pre-Teacher training* Architecture Projects Streaming live events Viz lab student films * Funded projects 5 Study Design Quasi-experimental pre-posttest control group research design study – 2 classes of Chem 101 students with same instructor (me) – ~ 240 students in each class – all assessments with HW worth 40/700 pts. Experimental group: 3 activities in SL (6wks) Control group: same 3 activities using two 2-D rotated images (SL screen shots) See blog: chemist-in-sl.blogspot.com 6 Study Design Blog: chemist-in-sl.blogspot.com 7 Pre-Testing Spatial Ability – Card Rotations Test (2-D) from ETS: paper Educational Testing Service http://www.ets.org/ – Purdue Spatial Visualization Test (3-D): online Bodner, George M, and Roland B Guay. “The Purdue Visualization of Rotations Test.” The Chemical Educator 2.4 (1997) : 1-17. Chemistry Content – VSEPR theory: online Science logic – Test of Logical Thinking (TOLT): online http://ken.tobinweb.net/Papers/TOLT.pdf 8 Pre-Testing On-line tests prepared by us with Qualtrics PSVT (20 items, 10 min) Free! Lots of research uses them! TOLT (10 items, 38 min) 9 Pre-Testing CRT – 20 items with 8 parts each – 10 items per page, 3 min per page – Students must identify if each figure, when compared to the main figure on the left, is the Same - S (rotated in the plane), or Different – D (flipped over and rotated) 10 Pre-Testing VSEPR test – developed by me – Tested on 3 faculty and ~100 past students – 36 questions, 4 parts, 45 min (angle, content knowledge, shape id, chemical examples of shape) – Reliability: 0.90 (Cronbach’s alpha) based on post test data 11 Post-Testing Spatial Ability – Card Rotations Test – Purdue Spatial Visualization Test Chemistry Content – VSEPR theory on-line test Presence questionnaire + Demographics – Included qualitative aspect HW & Laboratory module on VSEPR theory Exam questions - same for both groups 12 Intervention #1 The Molecule Game Experimental – Develop SL skills beyond SL &TAMU orientations (inventory, chat, interacting with objects, taking photos) – Begin seeing molecules in 3-D Control – Answer same questions from SL screen shots (2 views)13 Intervention #1 The Molecule Game Control – Students answer the same questions using SL screen shots (2 views) 14 Intervention #1 Feedback The Molecule Game 87% written directions were clear (N=71) 69% watched video & found it helpful (N=67) 86% MG is useful to learn SL skills (N=49) 85% MG ran smoothly (N=13) 15 Intervention #2 The Chemist as Artist Experimental – Develop more SL skills – Interact and draw 2 views each of 3 molecules in sandbox with photos (link, move, copy, rotate) ⇒ Control – Draw given 2 views of 3 molecules (5 different sets – screen shots)16 Intervention #2 The Chemist as Artist Control - Draw given 2 views of 3 molecules (5 different sets – screen shots) 17 Intervention #3 Tower of VSEPR Experimental – Rez 11 molecules – Measure bond angles, determine geometries, Lewis dot structures, draw structures Control – Do same homework from 2 views of each SL molecule (5 sets) 18 Intervention #3 Tower of VSEPR HW 19 Student Acceptance Student Issues Solutions – On-line test compliance gave pts/reminders – SL learning curve videos/ppt in SL/RL – not virtual world savvy SL/RL office hours – some think SL is creepy TAMU Island 20 Preliminary Results Before interventions, the control (C) and experimental (E) groups were the same as measured by: C (N=137) Mean (SD) E (N=153) Mean (SD) TOLT (max=10) 6.43 (2.10) 6.14 (2.24) PVRT (max=20) 12.36 (3.48) 11.86 (3.70) VSEPR Content Test (max=45) 7.08 (3.56) 7.39 (3.77) TEST 21 Preliminary Results The two groups were different on CRT pre-test but NOT post-test. The E group showed a small but significantly larger gain. CRT (max=160) Control Mean (SD) Experimental Mean (SD) Pretest 107.9 (30.4) 100.3 (27.9) 0.027 (2-tailed) Posttest 127.2 (29.5) 123.8 (22.5) 0.28 Gain (over 6 wks) p 19.3 (20.4) 23.5 (17.8) 0.029 (1-tailed) p<0.001 p<0.001 Cohen’s d=0.22 Cohen’s d=0.64 Cohen’s d=0.93 22 Preliminary Results PVRT – no significant differences were seen between the pretest and posttest for either group. Exam 3 score on VSEPR – no significant differences VSEPR HW and Laboratory – no significant differences VSEPR content test – Overall, no significant differences were seen on posttest Control: 18.41 ± 7.60 Experimental: 18.67 ± 8.73 23 Preliminary Results More on VSEPR test: On Part 1 (determining angle from drawing), the E group did significantly better (max=4) VSEPR Pt 1 (max=4) Control Mean (SD) Experimental Mean (SD) Pretest 1.01 (0.57) 1.07 (0.47) 0.26 (2-tailed) Posttest 1.74 (1.20) 2.01 (1.19) 0.03 (1-tailed) Cohen’s d=0.24 Gain (over 6 wks) p 0.74 (1.20) 0.94 (1.14) 0.075 (1-tailed) p=0.004 p<0.001 Cohen’s d=0.18 Cohen’s d=0.78 Cohen’s d=1.04 24 Preliminary Results Even more on VSEPR test: If you include all pre/post students, the results are even more significant, BUT On average, students are recognizing <2/4 of angles . VSEPR Pt 1 (max=4) Control Mean (SD) Experimental Mean (SD) Pretest 1.03 (0.58) 1.04 (0.51) 0.82 (2-tailed) Posttest 1.55 (1.17) 1.82 (1.19) 0.019 (2-tailed) Cohen’s d=0.24 Gain (over 6 wks) p 0.52 (1.17) 0.78 (1.16) 0.023 (2-tailed) P<0.001 p<0.001 Cohen’s d=0.22 Cohen’s d=0.56 Cohen’s d=0.85 25 Preliminary Results Let’s look at the questions one by one. Here is the question verbage: 26 Question 1 of 4 Gain-C (-0.04 ± 0.49; N=194) Gain-E (0.005 ± 0.44; N=209) p < 0.16 (1-tailed) 100 Percent 80 Confidence–Pre C=74% E=75% Confidence–Post C=77% E=82% 60 40 Control-Pre Control-Post Experiment-Pre 20 Experimental-Post 0 30o 45o 60o 90o 109o 120o 150o 180o Bond Angle 27 Question 2 of 4 Gain-C (0.25 ± 0.47) Gain-E (0.33 ± 0.49) 60 Confidence–Pre C=58% E=75% Confidence–Post C=63% E=60% 50 Percent p < 0.043 (1-tailed) 40 Confidence–Pre C=53% E=72% Confidence–Post C=75% E=76% Control-Pre 30 Control-Post 20 Experiment-Pre Experimental-Post 10 0 30o 45o 60o 90o 109o 120o 150o 180o Bond Angle 28 Question 3 of 4 Percent Gain-C (0.08± 0.44) Gain-E (0.15 ± 0.49) 45 40 35 30 25 20 15 10 5 0 Confidence–Pre C=46% E=57% Confidence–Post C=74% E=75% p < 0.064 (1-tailed) Confidence–Pre C=61% E=59% Confidence–Post C=65% E=62% Control-Pre Control-Post Experiment-Pre Experimental-Post 30o 45o 60o 90o 109o 120o 150o 180o Bond Angle 29 Question 4 of 4 Gain-C (0.23± 0.49) Gain-E (0.29 ± 0.47) p < 0.086 (1-tailed) 90 80 Confidence–Pre C=68% E=66% Confidence–Post C=70% E=68% 70 60 50 40 30 Confidence–Pre C=34% E=42% Confidence–Post C=75% E=74% Control-Pre Control-Post Experiment-Pre Experimental-Post 20 10 0 30o 45o 60o 90o 109o 120o 150o 180o 30 Comments Disheartening!! 31 More Preliminary Results We analyzed two questions posed to the Experimental group: 1. Is it a good idea to use Second Life in future chemistry classes? (Quantitative analysis) 2. Please explain your answer. (Qualitative analysis) 32 Is it a good idea to use Second Life in future classes? 35 30 Percentage N = 219 30% 25 23% 20 18% 18% 15 10 11% 5 0 Strongly Agree Agree Neutral Disagree Strongly Agree 33 Please Explain Your Answer…. Main Theme - Perceived Usefulness Perceived Usefulness 70 Percentage 60 1. Visualization (38%) 58% 2. Time Consumption (29%) 50 35% 40 3. Interactivity (17%) 30 20 7% 10 0 Yes Subthemes: No Unsure 4. Other methods such as text books, 2-D images, paper pencil were just as useful (16%) N = 100 Other themes: Perceived ease of use & gaming experience 34 Results - Perceived Usefulness Comments 1. It helped a lot with the VSEPR theory by allowing me to visualize the molecules so when I’m drawing them on paper I can see the molecule in my mind’s eye. This allowed me to draw 3D molecule faster and easier. 2. I found that it took a lot of time to learn how to manipulate the molecules in Second Life. 3. It allows you actually see and interact with a molecule 4. I feel it would be easier to lecture about this material and show students more examples. 35 Future Work/Questions Did my attempt to show the correct molecular shape by giving 2 views of a molecule to the control group skew the CRT results? Do students taking a regular general chem class increase their CRT scores naturally? If so, do they keep their higher CRT scores over time? Can we really assume that students can see that this angle is 90o, without training them? Not! 36 Acknowledgements Zahira and I thank all my students who had to endure all our assessments…. Thank you and any questions? 37