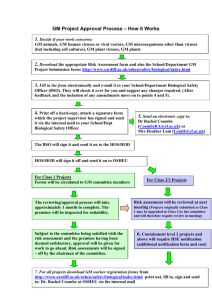
GMO Toolbox Talk
advertisement
Health and Safety Executive Genetically Modified Organisms (Contained Use) Regulations 2014 Implements 2009/41/EC (GM Contained Use Directive) What’s new in the 2014 Regulations? Sector description Containment Level Number of GM Centres Percentage CL1 342 57% CL2 179 30% CL3 71 12% CL4 6 1% Total 598 100% Breakdown of employers and premises by class of activity (1 Aug 2013) Background to consolidation Löfstedt review - ‘Reclaiming health and safety for all’ (November 2011) • Consolidation (4 sets of regulations into 1 consolidated regulations) • Reflect working practices, new technologies, simplify (e.g. duplication), remove unnecessary gold plating, involve/inform business of changes, evidence based policy Government committed to deliver by end 2014 • Maintain health and safety standards • Will come into force on 1 October 2014 What we needed to achieve Key objectives for the new regulations • • • • • Deliver the consolidation but not re-write (evolution rather than revolution) Take the opportunity to make regulations more risk based and proportionate Ensure any changes maintain the level of protection of human health and the environment (maintain health and safety standards) Where possible, remove barriers to development of a future single regulatory framework Apply the new rules from the Better Regulation Executive (e.g. impact assessment) Engagement with stakeholders Fact finding questionnaire circulated to flag up issues with regulations – scientists, safety professionals, unions, other government departments etc. Consulted other European regulators (European Enforcement Project) – Interpretation of directive – Alternative approaches taken Development of proposed changes – Used feedback to inform options and approaches Public consultation • • • • • Consultative Document 268 (CD268) published on HSE website Consultation ran between 28 October and 20 December 2013 (8 weeks) >5000 stakeholders alerted to CD268, from which ~800 stakeholders downloaded the consultation document and 42 stakeholders responded Proposals in two parts: – Part 1 – containment measures; – Part 2 – restructure & technical tidy up Overall support for the proposed changes – ~83% support for Part 1 – ~88% support for Part 2 Changes in 2014 Regulations Part 1 – containment measures - Several measures removed from the containment tables - Number of changes to specific measures at a particular containment level Change to source of advice on risk assessment Laboratory work Changes to containment measures • Removing duplication Measures removed from the table: – Disinfection procedures (66%) – Hand washing facilities (76%) • Bottom line – Requirement for both disinfection procedures and hand washing facilities remain (Reg 18(2) schedule 7) Changes to CL1 containment measures Measure 2000 regulations 2014 regulations Biohazard sign (89%) Required where RA shows required Not required Inactivation Required where Required of waste RA shows required (88%) Use of Required where RA Not required isolators shows required (90%) Changes to CL2 containment measures Measure 2000 regulations 2014 regulations Inward airflow (84%) Required where RA shows required Not required Not required Required where RA shows required Written procedures (92%) Changes to CL3 containment measures Measure 2000 regulations 2014 regulations Required Required except for activities where transmission does not occur via airborne route HEPA filters (67%) Required Required except for activities where transmission does not occur via airborne route Observation window (70%) Required Required where RA shows required Inward airflow (74%) Changes to CL4 containment measures Measure 2000 regulations 2014 regulations Requirement for incinerator on site (84%) Required to be on site Removed from the table Prescriptive requirement for Class III MSC (89%) Class III cabinet required Required and all procedures with infective materials required to be contained within a cabinet/enclosure Purpose built facility (90%) Required and required to be purpose built Required Key changes - Genetic modification safety committee (GMSC) • Requirement for competent advice on risk assessments • Person with expertise or GMSC for class 1 • • • GMSC for class 2 and above Workload of GMSC Broader remit Changes in 2014 Regulations Part 2 – restructure & technical tidy up • • • Layout and language Terminology Procedures Changes to format of the regulations • Layout and language – Modern legal language – Better delineation of requirements of users and competent authority – Key regulations to remain the same (e.g. risk assessment) – On-line version of public register version only • Terminology – Larger GMOs, contained use, user, person responsible Change to administrative arrangements • Administrative arrangements/procedures - Class 2 notification - full risk assessment to accompany notification - Emergency plan – risk based requirement - Appeals procedure – grounds for appeal retained but the procedure replaced with on-line guidance Guide to the Regulations (L29) • Redrafted the guide to the regulations to reflect the changes in the legislation • Redrafted to reflect better regulation principles for guidance • Restrict to explaining the meaning of the regulation – some technical content to be moved to Compendium of Guidance • Compendium revision is underway – the current on-line version does not reflect the changes in the 2014 regulations Changes to L29 • Key areas highlighted from consultation – waste inactivation, GMSC, significant changes, connected programmes, synthetic biology, consistency with COSHH • Institute for Safety in Technology and Research (ISTR) developing separate industry guidance on examples of significant change • • Electronic version only On-line community used to provide feedback on draft L29 Transitional arrangements • Transfer of notifications made under 2000 Regulations (including conditions, derogations) • Two possible exceptions where the risk classification may increase - GM centres should review their existing assessments in relation to use of isolators at CL1; and inward airflow at CL2 • Where risk class increases notification to HSE is required within 90 day transitional period – No fee – Contained use can continue if risks remain the same – Derogation requests at the same time Further information • HSE website contains the following information and links (http://www.hse.gov.uk/pubns/books/l29.htm) – Further details of changes – Revised version of L29 – guide to the regulations – Additional GM and biosafety guidance – Link to ISTR guidance on significant change