Density Powerpoint
advertisement

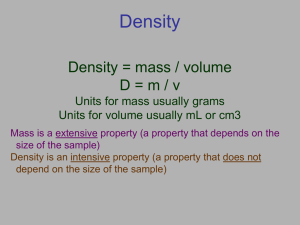
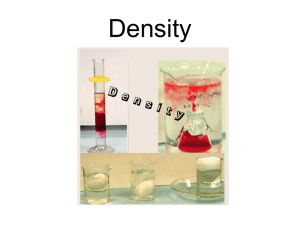
+ Density and Volume + Question: Which weighs more: A kilogram of feathers or a kilogram of iron How about: A 1 cm3 of feathers or a 1 cm3 of iron + cm3 = volume If you take the same masses of three different substances, obviously they will have the same masses If you take the same volume of three different substances, they will have different masses This is because those substances have different densities Wood Water Iron 1 cm3 1 cm3 1 cm3 0.50 g 1.00 g 8.00 g + So… what is density? Density is the proximity (closeness) of like atoms or molecules Density is a comparison of how much matter there is in a certain amount of space Density is a measurement of not just the “heaviness” of an object, but also how much space an object takes up!! All substances have density including liquids, solids, and gases Liquid Layers Which layer has the highest density? Which layer has the lowest density? Imagine that the liquids have the following densities: 10g/cm3 -- 3g/cm3 6g/cm3 -- 5g/cm3 Which number would go with which layer? Liquid Layers – Try on your own! Imagine that the liquids on the right have the following densities: 15g/cm3 10g/cm3 3g/cm3 9g/cm3 7g/cm3 12g/cm3 Match the colors to the correct densities. 3g/cm3 7g/cm3 9g/cm3 10g/cm3 12g/cm3 15g/cm3 + Which one is more dense? A local demonstration for the new school dress code is scheduled on 2 consecutive days in the town square Assume everyone has identical mass Which day is more dense? Day 1 Day 2 + Which one is more dense? Now which day is more dense? + Objects that float Why Does an Ice cube float and a marble sink? If the objects density is < 1.000 g/cm3 or g/mL, THEN IT WILL FLOAT!!!!! + Let’s try a density problem together Josh has a paper clip. It has a mass of 9g and a volume of 3cm3. What is its density? Christina also has an eraser. It has a mass of 3g, and a volume of 1cm3. What is its density? + Work on these problems with your neighbor. Peyton has a rock. The rock has a mass of 6g and a volume of 3cm3. What is the density of the rock? Matt has a gel pen. The gel pen has a mass of 8g and a volume of 2cm3. What is the density of the pen? + Now, try these on your own. Riley has a watch. It has a mass of 4g and a volume of 2cm3. What is the density of the watch? Mia has a wallet. It has a mass of 15g and a volume of 5cm3. What is the density of the wallet? + Now let’s try some harder ones…. You have a stone with a mass of 620 g and a volume of 753 cm3. Find the stone’s density. D (ρ) = M V M D V + More… A liquid has a density of 0.87 g/ml. What volume is occupied by 25 g of the liquid? (Hint: 1 cm3 = 1 mL) D (ρ) = M V M D V + …And One More An object has a volume of 825 cm3 and a density of 13.6 g/cm3. Find its mass in kilograms. D (ρ) = M V M D V + Review What is the formula for density? What happens if you pour together liquids that have different densities? Will the liquid on the top have the highest or lowest density? Will the liquid on the bottom have the highest or lowest density? + Super Scientist Question of the Day Jake has a book, a ruler, and a balance. How can Jake find the density of the book with the tools he has?