Photosynthesis Lab Powerpoint
advertisement

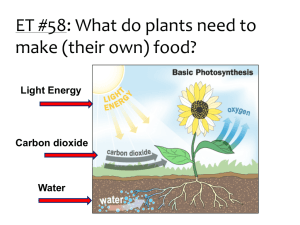
Tuesday, November 23, 2010 • Objective: Photosynthesis Lab! • Bellringer: Draw a Photosynthesis “KWL” Chart over both Tuesday and Wednesday for this week. – Today (Tuesday), you will fill in the K and W columns. – Tomorrow (Wednesday), you will fill in the L column. BELL RINGER Fill out the first two columns of the KWL chart. Leave the “L” column blank until tomorrow. 5 MINUTES K What do you know about photosynthesis? W What do you want to know about photosynthesis? L What did you learn about photosynthesis? BELL RINGER Fill out the first two columns of the KWL chart. Leave the “L” column blank until tomorrow. 4 MINUTES K What do you know about photosynthesis? W What do you want to know about photosynthesis? L What did you learn about photosynthesis? BELL RINGER Fill out the first two columns of the KWL chart. Leave the “L” column blank until tomorrow. 3 MINUTES K What do you know about photosynthesis? W What do you want to know about photosynthesis? L What did you learn about photosynthesis? BELL RINGER Fill out the first two columns of the KWL chart. Leave the “L” column blank until tomorrow. 2 MINUTES K What do you know about photosynthesis? W What do you want to know about photosynthesis? L What did you learn about photosynthesis? BELL RINGER Fill out the first two columns of the KWL chart. Leave the “L” column blank until tomorrow. 1 MINUTE K What do you know about photosynthesis? W What do you want to know about photosynthesis? L What did you learn about photosynthesis? TIME’S UP!!!!! Using Elodea to demonstrate Photosynthesis 6CO2 + 6H2O light C6H12O6 + 6O2 > How can we determine if photosynthesis occurs? • Bromothymol blue is a chemical that will be blue at pH around 7.6 and yellow-green at pH of 6 • CO2 can turn into carbonic acid when combined with water • If you blow through a straw into a solution of bromothymol blue, some CO2 will turn into Carbonic acid (so if enough CO2 is present in a bromothymol blue solution, it will have a yellow-green color.) • If an initial color of yellow-green turns blue, then what can you infer? On your lab worksheet… • What question are we trying to answer by completing this lab? • As a pair, make a hypothesis. Based on what you know about photosynthesis and what you have just learned about bromothymol blue, do you think the presence of light will affect photosynthesis and cause a change in the color of the bromothymol blue solution? Lab Set-up EL Elodea + Light ED Elodea + Dark L Light Control D Dark Control 1. Label your beaker with your Period and Group Number. 2. Label your 4 test tubes: EL, ED, L, and D 3. Carefully (SUPER SLOWLY) use your straw to blow air into the beaker. Continue until your solution turns a yellowish color. 4. Place each of your 2 Elodea pieces in the 2 test tubes labeled Elodea Light and Elodea Dark (1 piece per test tube). 5. Pour 10 mL of the bromothymol blue solution into each of the 4 tubes (enough to cover plants). 6. Cap each test tube. 7. Cover the test tubes labeled Elodea Dark and Dark Control with the aluminum foil (make sure the entire tube is covered so no light can get through). 8. Place all test tubes in the empty beaker, (make sure your beaker is labeled) and set the beaker on the window sill. 9. Answer the remaining questions for Day 1 on the lab hand out. Debrief/Wrap up • NOS – How does the lab demonstrate that science is tentative? – How does the lab demonstrate the concept of observation vs. inference? – Was there anything subjective about the lab? – Are there any other NOS categories that you can relate to this lab? • KOI – In this lab, did we test a hypothesis? – Was our procedure guided by a question? – Give an example of how our procedure could have influenced our results. – Did we all get the exact same results? Why or why not? – Were our conclusions consistent with our data? – Explain how in this lab data and evidence are not the same.