Bellwork
advertisement

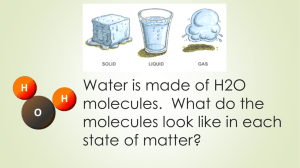
Bellwork – 01/22/14 1] Evaporation of rubbing alcohol – physical or chemical change? 2] Would you add or remove energy ( to/from the water ) to do this? ** What is the source of the energy? Bellwork – 01/22/14 1] Evaporation of water – physical or chemical change? Physical 2] Would you add or remove energy ( to/from the water ) to do this? Add Grab notebooks/study guides at front of room Bellwork: What are the four states of matter? Bellwork: What are the four states of matter? Solid, Liquid, Gas, Plasma TN Standards • CLE.3202.Inq.2 – Recognize that science is a progressive endeavor that reevaluates and extends what is already accepted • CLE.3202.Inq.3 – Use appropriate tools and technology to collect precise and accurate data • CLE.3202.Inq.6 – Communicate and defend scientific findings • CLE.3202.1.4 – Investigate chemical and physical changes How Does Matter Change States? Essential Questions: • 1] What makes up matter? • 2] What kind of energy do all particles of matter have? • 3] What happens when a substance changes from one state of matter to another? • 4] What happens to mass and energy during physical and chemical changes? Demonstration 1 • Have cotton balls with rubbing alcohol added • Student in the front of each row come to the front and grab enough cotton balls for row • Each student rub the alcohol on their arm ( skin ) • What happens? What kind of sensations do you feel? • Why is this happening? What is Happening? Evaporation • Your body heat gets transferred to the rubbing alcohol and it evaporates • This gives that part of your skin a “cooling” sensation Some Changes Absorb Energy • Energy is added when water ( or any substance ): • Evaporates • Melts • Sublimates ( dry ice ) Some Changes Release Energy • Energy is removed when water ( or any substance ): • Condensation • Freezing Sweating – Cools Your Body • So which of the five state changes does sweating represent? • Is energy released or absorbed when the sweating process occurs? • How does a dog sweat? Demonstration 2 ( ongoing ) • Will start boiling water in the back of room • Will take a few notes while water boils • Once water boils, will add ~butter to it Changes of State • The identity of a substance does not change when the state changes but the energy changes Active Demonstration • I will guide you through this • We will have three groups • Each group will act like either particles in a gas, liquid, or solid ( one at a time ) • Each group will stand up and act out this role Active Demonstration - Results • Solids: particles vibrate in place • Liquids: particles move faster than in solid so they spread out more • Gas: particles move very fast so they are really far apart • Can you walk through ice, water, humid air? Kinetic Theory of Matter • Matter is made of atoms and molecules • These act like tiny particles that are ALWAYS in motion • Particles move faster at higher temperatures • Bigger particles move slower than smaller ones AT THE SAME TEMPERATURE Review States of Matter ( Volume & Shape ) • Solids – definite shape and volume? • Liquids – definite shape and volume? • Gas – definite shape and volume? Review States of Matter ( Volume & Shape ) • Solids – definite shape and volume? – BOTH • Liquids – definite shape and volume? – ONLY VOLUME • Gas – definite shape and volume? – NEITHER Solid, Liquid, Gas Temperature • Particles in all states of matter have kinetic energy • Temperature is a measure of the average kinetic energy of particles in an object Conservation of Mass & Energy • Mass and Energy are both CONSERVED ALWAYS • Mass/Energy is neither created nor destroyed • This applies to state-changes also Demonstration 2 ( Butter ) • Of course you know this, but what happens when the butter is added to the hot water? • Why does this happen? • How does energy ( of both the water and the butter ) change during this process? • What happens to the particles in both the water & butter ( speed up or slow down ) ? Practice - Study Guides • Pg 10 ( #s 1, 2, 5 ) • Pg. 11 ( #s 1, 2, 3, 4 ) Challenge – In Your Notes • Fog: which of the 5 state-changes does fog represent? • When/Where does fog form • Is energy absorbed or released when fog forms? • Is energy and mass conserved when fog forms? Exit Pass • On a Whiteboard, make a table with 3 columns and 4 rows • list four ( 4 ) common, everyday examples of statechanges in first column • Put which of the 5 state changes they are in middle • List whether energy is released or absorbed on right Exit Pass • Draw a picture that indicates a state-change ( you choose !! ) then label which of the 5 statechanges it is and whether energy is added or removed [from the substance changing].