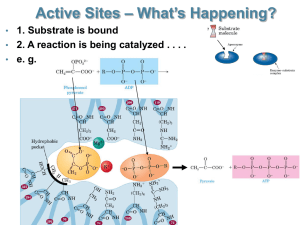
Non-competitive inhibition
advertisement

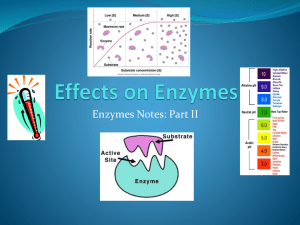
Overview • Enzymes are specialized proteins that function as catalysts to increase the rate of biochemical reactions. By interacting with substrates (reactant molecules upon which an enzyme acts), enzymes catalyze chemical reactions involved in the biosynthesis of many cellular products. Enzymes derive their name from Greek, in which the term enzyme means “in yeast” and was mainly used to distinguish the whole microorganism, such as yeast (“organized ferments”), from that of extracts of the whole microorganisms (“unorganized ferments”). The implication was that enzymes were unorganized ferments in yeast. Although the vast majority of enzymes are proteins, certain nucleic acids (RNAs) also possess enzymatic activity (i.e., ribozymes). Enzymes are the most efficient catalysts known in nature, because they have the ability to increase reaction rates by enormous factors. Like all catalysts, enzymes have the ability to lower the activation energy of reactions, and the tremendous catalytic power they possess results from their inherent ability to provide stabilization to the reacting molecules at their activated complex states. Classification Enzymes have been classified on the type of reaction catalyzed, and six major classes (families) of enzymes, numbered from 1 to 6, have been assigned by the Enzyme Commission (EC) of the International Union of Biochemistry and Molecular Biology (7). These classes are as follows: 1) oxidoreductases (e.g., dehydro-genases); 2) transferases (group transfer enzymes; e.g.,kinases); 3) hydrolases (hydrolytic reactions; e.g., ester-ases); 4) lyases (formation or removal of double bonds; e.g., hydratase— addition of water across a double bond); 5) isomerases (e.g., mutarotation of glucose by mutases); and 6) ligases (joining of two substrates at the expense of energy, also referred to as synthetases). All discovered enzymes are identified by the prefix EC followed by an Arabic numeral based on the major class of reaction catalyzed, as indicated earlier. Furthermore, this is followed by a series of three more Arabic numerals, which indicate the subclass (functionality), sub-sub class (specific bond type), and serial number of the enzyme in that class, respectively. For example, the enzyme acetylcholinesterase has been given the following assignment: EC 3.1.1.7. As can been seen in this example, the numeral 3 indicates that this enzyme belongs to the family of hydrolases, the first 1 indicates that the nature of the bond being hydrolyzed is an ester, the second 1 indicates that specific ester bond is a carboxylic acid ester, and the last number is the serial number of this enzyme in this subclass. GENERAL CONCEPTS OF ENZYME INHIBITION The body is composed of thousands of different enzymes, many of them acting in concert to maintain homeostasis. Although disease states may arise from the malfunc-tioning of a particular enzyme, or the introduction of a foreign enzyme through infection by microorganisms, inhibiting a specific enzyme to alleviate a disease state is a challenging process. Most bodily functions occur through a cascade of enzymatic systems, and it becomes extremely difficult to design a drug molecule that can selectively inhibit an enzyme and result in a therapeutic benefit. However, to address this problem, the basic mechanism of enzyme action needs to be understood. Once knowledge of a particular enzymatic pathway is determined and the mechanism and kinetics are worked out, the challenge is then to design a suitable inhibitor that is selectively used by the enzyme causing its inhibition. Competitive inhibition is a form of enzyme inhibition where binding of the inhibitor to the active site on the enzyme prevents binding of the substrate and vice versa Uncompetitive inhibition, also known as anti-competitive inhibition, takes place when an enzyme inhibitor binds only to the complex formed between the enzyme and the substrate (the E-S complex). Non-competitive inhibition is a type of enzyme inhibition where the inhibitor reduces the activity of the enzyme and binds equally well to the enzyme whether or not it has already bound the substrate Reversible Enzyme Inhibition • Reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both Irreversible enzyme inhibition • Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. How to understand the type of inhibition: Enzyme kinetics Irreversible inhibitor