Lecture 1 - Quantum Chemistry Laboratory
advertisement
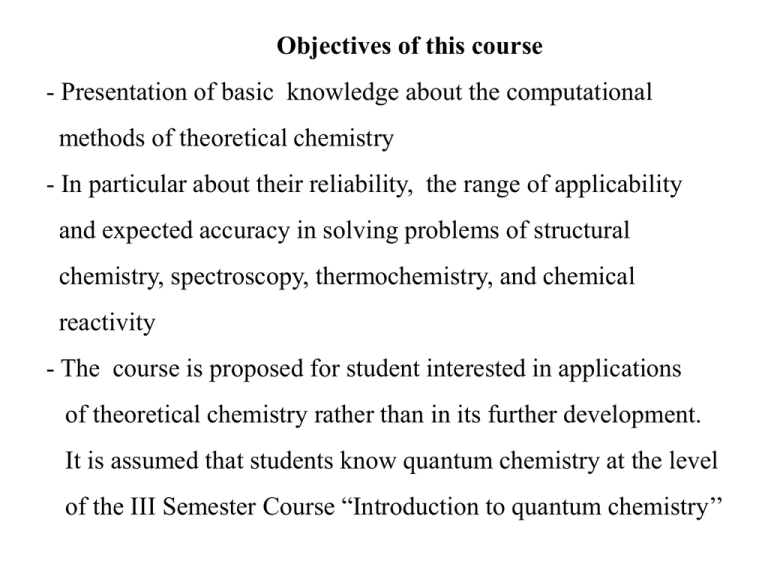
Objectives of this course - Presentation of basic knowledge about the computational methods of theoretical chemistry - In particular about their reliability, the range of applicability and expected accuracy in solving problems of structural chemistry, spectroscopy, thermochemistry, and chemical reactivity - The course is proposed for student interested in applications of theoretical chemistry rather than in its further development. It is assumed that students know quantum chemistry at the level of the III Semester Course “Introduction to quantum chemistry’’ The objectives of theoretical chemistry - Prediction of properties of single molecules, in particular: - molecular structure (geometry – bond lengths, angles) - molecular charge distribution (dipole, quadrupole moments) - energetics: bond dissociation energies, conformation energies, barriers, activation energies, reaction energies - spectra (rotational, vibrational, electronic, NMR, EPR,... - electric and magnetic properties of molecules: polarizability, magnetic susceptibility The objectives of theoretical chemistry, continued - Prediction of properties of molecular aggregates, supramolecular and macroscopic systems, in particular: - intermolecular interactions - thermodynamic properties and functions (like entropy) and chemical equilibrium constants - properties of liquids and solids - relaxation processes - characteristics of phase transitions - rates of chemical reactions in the gas, liquid and solid phase - mechanisms of catalytic reactions Parts of theoretical chemistry - Quantum Chemistry - electronic structure theory - Born-Oppenheimer approximation and the concept of the Potential Energy Surface (PES) or curve - theory of nuclear (rovibrational) dynamics in molecules - theory of molecular collisions and reactions - theory of nonadiabatic processes - Statistical thermodynamics and mechanics - analytic methods (classical and quantum) - computer simulation methods - Monte Carlo methods (classical and quantum) and classical molecular dynamics Quantum Mechanics - non-relativistic (Schrodinger-Coulomb equation) - relativisitc (Dirac-Coulomb equation) - quantum field theory (Quantum ElectroDynamics, QED) Example of achievable accuracy – dissociation energy (in 1/cm) of the chemical bond hydrogen 36118.7978(2) 36118.2659(3) 36118.0695(9) 36118.0696(4) deuterium 36749.0910(2) 36748.5634(3) 36748.3633(9) 36748.3629(6) theory Schrodinger-Coulomb relativistic QED experiment Born-Oppenheimer approximation for diatomic molecules (PEC) Electronic Schrodinger equation Nuclear Schrodinger equation - rotations - J quantum number (rigid rotor model) - oscilations – v quantum number (harmonic oscillator model) Potential V(R) for nuclear motion in a diatomic molecule Harmonic oscilator potential Wave functions of the harmonic oscillator Effect of zero-point vibrations - ZPE Dissociation energy of a diatomic molecule: A-B A + B E(A) + E(B) ZPE E(AB) (lowest point) Two definitions: Electronic binding energy (well depth): De = E(A) + E(B) - E(AB@Req) Dissociation energy: D0 = E(A) + E(B) - [E(AB@Req + ZPE] = De - ZPE Born-Oppenheimer approximation for polyatomics (PES) Electronic Schrodinger equation Nuclear Schrodinger equation - rotations - J quantum number (rigid rotor model) - oscilations – v quantum numbers (harmonic oscillator model) - tunelling motions – for floppy molecules (ammonia moleucle) Three-atom molecule H2O N=3 # of deg. freed. = 3N-6 = 3 r2 H1 O r1 H2 Stationary points on PEC or PES Minima andmaxima in 1-D f(x) minimum: f’(x0)=0 f”(x0)>0 maximum: f’(x0)=0 f”(x0)<0 example: f = ax2 + bx + c f’ = 2ax + b f” = 2a a > 0 parabola - minimum; a<0 parabola - maximum (inflection points – less interesting) Similarly for PES’s – functions in 3N-6 dimensions: PES = E(q1, q2, q3, …, q3N-6(5) ) In a stationary point: E 0 qi Derivative of energy - gradient To locate stationary points on PES we must find points, where all gradients vanish. To distinguish minima and maxima ona has to compute the matrix of the second derivatives – the Hessian 2 E 2 2q1 E q q 2 1 2 E q3q1 2 E q42q1 E qnq1 2E q1q2 2E q22 2E q3q2 2E q4q2 2E qnq2 2E q1q3 2E q2q3 2E q32 2E q4q3 2E qnq3 2E q1q4 2E q2q4 2E q3q4 2E q42 2E qnq4 2 E q1qn 2 E q2qn 2 E q3qn 2 E q4qn 2 E qn2 n = 3N-6(5) 2 E 2 2q1 E q q 2 1 2 Hessian= E q3q1 .. . 2 E qnq1 2E q1q2 2E q22 2E q3q2 .. . 2E qnq2 2E 2 E … q1q3 q1qn 2 E … 2 E q2q3 q2qn 2 E … 2 E q32 q3qn .. . . 2 E . . q4qn 2 E … 2 E qnq3 qn2 Hessian is diagonalized and we look at its eigenvalues When all are positive we have a minimum Hessian diagonalized! 2 E 2 Q1 0 New coordinates 0 0 0 0 0 0 2E Q22 0 0 2E Q32 0 0 0 0 0 0 2E Q32 0 0 0 0 2 E Qn2 Eigenvalues of the Hessian Criteria Minimum: Saddle points: Maximum: All eigenvalues of All eigenvalues of the All eigenvalues of the Hessian are Hessian are positive the Hessian are positive except for one negatiove Minimum on PES – equilibrium geometry Saddle point on PES - transition state (a pass between two minima), reaction barrier, barier separating konformers Equilibrium geometry = locate minimum on PES Transition state geometry = locate a saddle point on PES Energy Profile = calculate cross-section of PES along one coordinate How is potential energy minimized (minimum located at the PES)? We know that in a minimum the first derivatives of energy (the gradient) is zero Start from an input structure (a point on PES) evaluate gradient at this point (a vector) go in the direction of the steepest descent (given by the gradient vector) as long as the energy decreases. when the energy stops to decrease compute the gradient again and repeat the procedure when the gradient reaches zero you are at the minimum (optimized structure and the equilibrium energy)