Class PowerPoint on Calorimetry

71 Days Until the Harvard
Westlake Final
• Catalyst:
1. What is entropy?
2. What is the triple point on a phase diagram?
3. What is the critical point on a phase diagram?
End
Today’s Learning Target
• 7.6 – Using calorimetry data, I can utilize my knowledge of temperature change and specific heats to calculate the amount of heat transferred for a reaction .
Today’s Focus Question
• How do we determine the amount of calories in Flamin’ Hot Cheetos?
Specific Heat
• The amount of energy that is transferred to a material is dependent on the nature of the material receiving the energy.
• Specific Heat – The amount of energy required to raise the temperature of one gram of substance one degree Celsius
• Represent by the symbol C p
Calorimetry
• Calorimetry is the science of measuring the heat of a chemical reaction .
q
m
c p
T
• q represents the heat gained/released
• C p is the specific heat
• ΔT = Final Temperature – Initial Temp.
• If we can measure the mass and temperature, then we can determine the heat gained/lost in a reaction
Class Example
• A 4.0 g sample of glass is heated from 274
K to 314 K. Glass has a specific heat of
0.20 J/(g x K). How much heat is gained during this heating?
Table Talk
• The specific heat of copper is 0.4 J/ o C.
How much heat is needed to change the temperature of a 30 g sample of copper from 20.0 o C to 60.0 o C?
Stop and Jot
• Determine the specific heat of a material if a 35 g sample absorbed 96 J as it was heated from 293 K to 313 K.
Calories in Flamin’ Cheetos
• Q can be measured in calories
• Food scientists measure the calories contained within food by measuring the amount of energy it takes to raise 1 g of water 1 o C.
• 1000 calories = 1 C alorie
• 1 serving of Cheetos has 140 Calories
SUMMARIZE
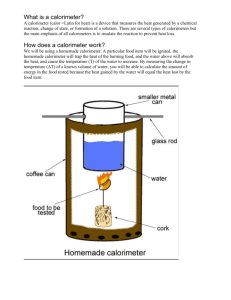
Lab Intro
• Info about lab procedures and equipment.
Lab Work Time
Lab Calculations
Work Time
• Begin working on your “Homework 7.3”
• There will be an exit slip after this activity
Learning Log Assessment
Rate yourself 1 – 4 on LTs 7.6
Exit Slip
1. I have a sample of water and I increase the temperature from 10.0 o C to 40 o C. The specific heat of water is 4.18 J/(g x o C).
Calculate the heat absorbed for this process.
Learning Log Assessment
Using your exit slip score, re-rate yourself on LTs 7.6
Closing Time
• Homework 7.3 – Calorimetry Calculations
• Rough Draft of Lab Report DUE
TOMORROW