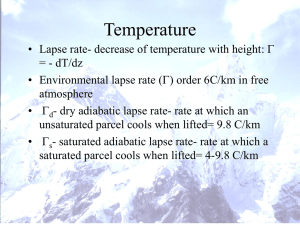
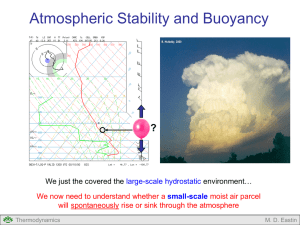
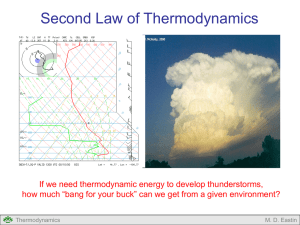
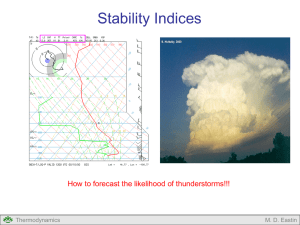
Lecture #4: Adiabatic Processes
advertisement

Adiabatic Processes 1000 mb How can the first law really help me forecast thunderstorms? Thermodynamics M. D. Eastin Adiabatic Processes Outline: Review of The First Law of Thermodynamics Adiabatic Processes Poisson’s Relation Applications Potential Temperature Applications Dry Adiabatic Lapse Rate Applications Thermodynamics M. D. Eastin First Law of Thermodynamics Statement of Energy Balance / Conservation: • Energy in = Energy out • Heat in = Heat out dq cvdT pdα Heating Sensible heating Latent heating Evaporational cooling Radiational heating Radiational cooling Thermodynamics Change in Internal Energy Work Done Expansion Compression M. D. Eastin Forms of the First Law of Thermodynamics For a gas of mass m For unit mass dQ dU dW dq du dw dQ dU pdV dq du pd dQ cvdT pdV dq cvdT pd dQ cpdT Vdp dq cpdT dp cp cv nR* cp c v R d where: p = pressure V = volume T = temperature α = specific volume U = internal energy W = work Q or q = heat energy n = number of moles cv = specific heat at constant volume (717 J kg-1 K-1) cp = specific heat at constant pressure (1004 J kg-1 K-1) Rd = gas constant for dry air (287 J kg-1 K-1) R* = universal gas constant (8.3143 J K-1 mol-1) Thermodynamics M. D. Eastin Types of Processes Isothermal Processes: • Transformations at constant temperature (dT = 0) Isochoric Processes: • Transformations at constant volume (dV = 0 or dα = 0) Isobaric Processes: • Transformations at constant pressure (dp = 0) Adiabatic processes: • Transformations without the exchange of heat between the environment and the system (dQ = 0 or dq = 0) Thermodynamics M. D. Eastin Adiabatic Processes Basic Idea: • No heat is added to or taken from the system which we assume to be an air parcel dq cvdT pdα 0 Parcel dq cpdT dp 0 • Changes in temperature result from either expansion or contraction • Many atmospheric processes are “dry adiabatic” • We shall see that dry adiabatic process play a large role in deep convective processes • Vertical motions • Thermals Thermodynamics M. D. Eastin Adiabatic Processes P-V Diagrams: Isobar p i Isochor Adiabat Isotherm f V Thermodynamics M. D. Eastin Poisson’s* Relation A Relationship between Temperature and Pressure: • Begin with: cpdT dp • Substitute for “α” using the Ideal Gas Law and rearrange: • Integrate the equation: pα R d T dT R d dp T cp p Tfinal Tinitial * NOT pronounced like “Poison” Thermodynamics Adiabatic Form of the First Law Rd dT T cp p final p intital dp p See: http://en.wikipedia.org/wiki/Simeon_Poisson M. D. Eastin Poisson’s Relation A Relationship between Pressure and Temperature: • After Integrating the equation: Tfinal Rd pfinal ln ln Tinitial cp pinitial • After some simple algebra: p final Tfinal Tinitial pinitial Rd cp Tfinal p final Tinitial pinitial Rd cp • Relates the initial conditions of temperature and pressure to the final temperature and pressure Thermodynamics M. D. Eastin Applications of Poisson’s Relation Tfinal p final Tinitial pinitial Rd cp Example: Cabin Pressurization • Most jet aircraft are pressurized to 8,000 ft (or 770 mb). If the outside air temperature at a cruising altitude of 30,000 feet (300 mb) is -40ºC, what is the temperature inside the cabin? Thermodynamics M. D. Eastin Applications of Poisson’s Relation Tfinal p final Tinitial pinitial Rd cp Example: Cabin Pressurization • Most jet aircraft are pressurized to 8,000 ft (or 770 mb). If the outside air temperature at a cruising altitude of 30,000 feet (300 mb) is -40ºC, what is the temperature inside the cabin? pinitial = 300 mb pfinal = 770 mb Rd = 287 J / kg K cp = 1004 J / kg K Tinitial = -40ºC = 233K Tfinal = ??? Thermodynamics M. D. Eastin Applications of Poisson’s Relation Example: Cabin Pressurization • Most jet aircraft are pressurized to 8,000 ft (or 770 mb). If the outside air temperature at cruising altitude of 30,000 feet (300 mb) is -40ºC, what is the temperature inside the cabin? pinitial = 300 mb pfinal = 770 mb Rd = 287 J / kg K cp = 1004 J / kg K Tinitial = -40ºC = 233K Tfinal 770mb 233K 300mb 287 1004 Tfinal 305K Tfinal 32C Thermodynamics M. D. Eastin Applications of Poisson’s Relation Comparing Temperatures at different Altitudes: Are they relatively warmer or cooler? • Bring the two parcels to the same level • Compress 300 mb air to 600 mb 300 mb Tfinal p final Tinitial pinitial Thermodynamics Rd cp 600 mb -37oC 2oC M. D. Eastin Applications of Poisson’s Relation Comparing Temperatures at different Altitudes: Are they relatively warmer or cooler? Tfinal p final Tinitial pinitial Rd cp pinitial = 300 mb pfinal = 600 mb Tinitial = -37ºC = 236 K Tfinal = 288 K = 15ºC 300 mb -37oC 600 mb 2oC 15oC Note: We could we have chosen to expand the 600 mb parcel to 300 mb for the comparison Thermodynamics M. D. Eastin Potential Temperature Special form of Poisson’s Relation: Compress all air parcels to 1000 mb • Provides a “standard” • Avoids using an arbitrary pressure level • Define Tfinal = θ • θ is the potential temperature 1000mb θ Tinitial pinitial p0 θ T p Rd Rd cp cp 1000 mb where: p0 = 1000 mb Thermodynamics M. D. Eastin Applications of Potential Temperature Comparing Temperatures at different Altitudes: An aircraft flies over the same location at two different altitudes and makes measurements of pressure and temperature within air parcels at each altitude: Air parcel #1: Air Parcel #2: p = 900 mb T = 21ºC p = 700 mb T = 0.6ºC p0 θ T p Rd cp Which parcel is relatively colder? warmer? Thermodynamics M. D. Eastin Applications of Potential Temperature Comparing Temperatures at different Altitudes: Air Parcel #1: p = 900 mb T = 21ºC = 294 K 1000mb θ 294K 900mb 0.286 θ 303K Air Parcel #2: p = 700 mb T = 0.6ºC = 273.6 K 1000mb θ 273.6K 700mb 0.286 θ 303K The parcels have the same potential temperature! Are we measuring the same air parcel at two different levels? Thermodynamics M. D. Eastin Applications of Potential Temperature Potential Temperature Conservation: • Air parcels undergoing adiabatic transformations maintain a constant potential temperature (θ) • During adiabatic ascent (expansion) the parcel’s temperature must decrease in order to preserve the parcel’s potential temperature • During adiabatic descent (compression) the parcel’s temperature must increase in order to preserve the parcel’s potential temperature Constant θ Thermodynamics M. D. Eastin Applications of Potential Temperature Potential Temperature as an Air Parcel Tracer: • Therefore, under dry adiabatic conditions, potential temperature can be used as a tracer of air motions Constant θ Thermodynamics Constant θ • Track air parcels moving up and down (thermals) • Track air parcels moving horizontally (advection) M. D. Eastin Dry Adiabatic Lapse Rate How does Temperature change with Height for a Rising Thermal? • Potential temperature is a function of pressure and temperature: θ(p,T) • We know the relationship between pressure (p) and altitude (z): dp g dz Hydrostatic Relation (more on this later) • We can use this hydrostatic relation and the adiabatic form of the first law to obtain a relationship between temperature and height when potential temperature is conserved (dry adiabatic lapse rate) cpdT dp Thermodynamics Adiabatic Form of the First Law z Dry Adiabatic Lapse Rate? T M. D. Eastin Dry Adiabatic Lapse Rate How does Temperature change with Height for a Rising Thermal? • Begin with the first law: • Substitute for “α” using the Ideal Gas Law and rearrange: • Divide each side by “dz”: • Substitute for “dp/dz” using the hydrostatic relation and re-arrange: Thermodynamics cpdT dp dT R d dp T cp p 1 dT R d 1 dp T dz c p p dz dp g dz T Rd g dT dz p cp M. D. Eastin Dry Adiabatic Lapse Rate How does Temperature change with Height for a Rising Thermal? • Substitute for “ρ” using the Ideal Gas Law and cancel terms: T Rd g dT dz p cp p R dT dT g dz cp • We have arrived at the Dry Adiabatic Lapse Rate (Γd): dT g d 9.8C / km dz cp Thermodynamics M. D. Eastin Application of the Dry Adiabatic Lapse Rate Example: Temperature Change within a Rising Thermal • A parcel originating at the surface (z = 0 m, T = 25ºC) rises to the top of the mixed boundary layer (z = 800 m). What is the parcel’s new air temperature? dT 9.8C / km dz Tfinal (9.8C / km) dz Tinitial Tfinal 9.8 * 0.8 25 Tfinal 17.2C Mixed Layer Constant θ Thermodynamics M. D. Eastin Adiabatic Processes Summary: • Review of The First Law of Thermodynamics • Adiabatic Processes • Poisson’s Relation • Applications • Potential Temperature • Applications • Dry Adiabatic Lapse Rate • Applications Thermodynamics M. D. Eastin References Petty, G. W., 2008: A First Course in Atmospheric Thermodynamics, Sundog Publishing, 336 pp. Tsonis, A. A., 2007: An Introduction to Atmospheric Thermodynamics, Cambridge Press, 197 pp. Wallace, J. M., and P. V. Hobbs, 1977: Atmospheric Science: An Introductory Survey, Academic Press, New York, 467 pp. Thermodynamics M. D. Eastin