Presentazione di PowerPoint
advertisement

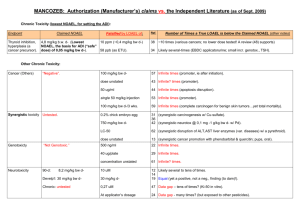
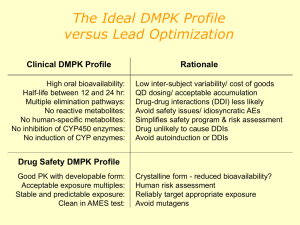
CONFERENCE ON “ FOOD ADDITIVES : SAFETY IN USE AND CONSUMER CONCERNS“ JOMO KENYATTA UNIVERSITY OF AGRICULTURE AND TECHNOLOGY NAIROBI , 24 JUNE 2014 HAZARD is the potencial capacity of producing harm. is proportional to both the and the . RISK ANALYSIS • Food additives are highly regulated at global level. (i.e. EFSA, FAs etc. etc.) • No food additive can be used without safety assessment and approval • Safety assessment is undertaken PRIOR to approval • The applicant (industry) provides the safety data, which have to be performed to defined quality standards (GLP, QA, OECD, UE guidelines……) • The assessment Panels – which include scientists and regulators with a wide range of expertise – are responsible for safety assessments Hazard identification Inherent biological activity Hazard assessment Assessment of relevance for humans Dose-response analysis EXPOSURE ASSESSMENT Active principle Dose of food additives Dose in individuals Dose in special population groups Max/min chronically/occasionally HAZARD IDENTIFICATION Identification of adverse health effects In silico methodologies In vitro toxicology data Animal-based toxicological studies Human observation HAZARD ASSESSMENT Quantification of adverse health effects Kinetic variability Dynamic variability Mode/mechanism of action Selection of critical data Dose-response for critical effect RISK CHARACTERISATION Principle 1 Risk management should follow a structured approach Principle 2 Protection of human health should be the primary consideration in risk management decisions Principle 3 Risk management decisions and practices should be transparent Principle 4 Determination of risk assessment policy should be included as a specific component of risk management Principle 5 Risk management should ensure the scientific integrity of the risk assessment process by maintaining the functional separation or risk assessment and risk management Principle 6 Risk management decisions should include clear, interactive communication with consumers and other interested parties in all aspects of the process Principle 7 Risk management should be a continuing process that takes into account all newly generated data in the evaluation and review of risk management decisions Reference points (RPs) in toxicology studies used to calculate a safe level for human intake: Benchmark Dose (BMD). Toxicant and/or NON Genotoxic Carcinogen Toxicants Dietary supplements Botanicals – Herbs Contaminants • ADI (Acceptable Daily Intake) • ARfD (Acute Reference Dose) • TMDI (Tolerable Maximum daily Intake) • XYZ ……………………………………………………… etc. etc ADI represents the amount of a food additive, a pesticide or a veterinary drug residue, expressed on a body weight basis, that can be ingested daily over a lifetime without appreciable health risk. ARfD represents the amount of a pesticide, expressed on a body weight basis, that can be ingested over a short period of time (one day) without appreciable health risk T(M)DI represents permissible human daily exposure to those contaminants, expressed on a body weight basis, unavoidably associated with the consumption of nutritious foods. ALLOCATION ADI – ARfD -TMDI– xxz….. TOXICOLOGICAL PROTOCOL Absorption LD50 oral Mutagenesis Distribution LD50 dermal Clastogenesis Metabolism LC50 inhalation Aneuploidy Excretion Skin irritation Eye irritation Skin sensitization Teratogenicity tests (Rat-Rabbit) Two generation reproductive toxicity Mouse 90 day toxicity Rat 90 day toxicity Dog 90 day toxicity Dog 1 year toxicity Mouse 18 months Rat 104 weeks • The greatest concentration or amount of an agent, found by study or observation that causes detectable, usually adverse (or toxic?) alteration of morphology, functional capacity, growth, development or lifespan of the target HUMANS sensitive subjects HUMANS population average ARfD AOEL 1 10 dose mg/kg bw 100 ADI = NOAEL SF ADI = Admissible Daily Intake mg/kg b.w. NOAEL = No Observed Adverse Effect Level (mg/kg b.w.) SF = Safety Factor (10, 100, n) Differences 10 Interindividual Differences 10 Interspecies -∞ Log Concentration +∞ Exposure assessment is a key element of risk assessment and a tool for risk management It’s theoretically simple but practically complex due to data deficiencies Examples of exposure models: ILSI Europe’s ‘ GUIDEA ‘ and FACET • Which substances are present in what amounts in a given food/diet: including information concerning factors influencing their levels and qualities such as bioavailability • How much of the foods containing these substances are consumed and what is the consumption of potentially relevant risk groups, including high users? • What are the conditions and the probabilities of consuming occasionally or regularly high amounts of such foods which at the same time contain high levels of the substance(s) in question? : Regulated Maximum Levels (MLs) in the EU for: mycotoxins (aflatoxins, ochratoxin A, patulin, deoxynivalenol, zearalenone, fumonisins, T2 and HT-2-toxin) metals (cadmium, lead, mercury and inorganic tin) dioxins and dioxin-like PCBs, 3-MCPD, polycyclic aromatic hydrocarbons (benz(o)pyrene). Manufacturer’s Use Levels Total Diet Survey ( TDS ) determines levels of various contaminants and nutrients in foods. Duplicate Diets Test persons consume their ordinary diet, but for subsequent analysis, they also prepare a duplicate portion of all food products as prepared, served and consumed. Individual Food Diary Records interviews. Household Budget Surveys ( HBS ) national surveys mainly focusing on consumption expenditure. • Methodologies to integrate food consumption, fate and chemical concentration to make the best estimate of exposure. Concentration of chemical in diet Weight of diet consumed daily Body weight (60 kg ) Exposure = mg/kg body weight/day Sampling and Analysis Temporal- extrapolation to lifetime exposure Under/Over reporting Representativeness of population sample Other sources of exposure eg. supplements , medicines Coding system not specific enough Portion size Processed food Consumer Loyalty Quantity of food consumed Distribution of concentrations Usage level of the Additive in food Presence probability Occurrence of the Additive in food INTAKE Specific Codification System ILSI EUROPE http://www.ilsi-guidea.org/index.php?title=Main_Page PERCEIVED RISKS (media) REAL RISK (WHO) Nutritional Deficiencies Food Additives Bacterial Intoxications Nutritional Deficiencies Biological Toxins Bacterial Intoxications Biological Toxins Food Additives ABSORPTION GENOTOXICITY In vitro testing TOXICITY (28-day/90-day study) TRIGGERS FOR CONSIDERING TIER 2 Toxicity in the 28/90-day study Guidance for submission for food additive evaluations EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) - EFSA Journal 2012;10(7):2760 Systemic availability Genotoxicity in vitro ADME Single dose GENOTOXICITY In vivo testing TOXICITY (stand alone or combined) Chronic toxicity Carcinogenicity REPRODUCTIVE & DEVELOPMENTAL TOXICITY Extended One–Generation Reproduction Toxicity Study PRENATAL DEVELOPMENTAL TOXICITY (Teratogenicity) TRIGGERS FOR CONSIDERING TIER 3 Bioaccumulation Positive in vivo genotoxicity Chronic toxicity/Carcinogenicity Reproductive & developmental toxicity Guidance for submission for food additive evaluations EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) - EFSA Journal 2012;10(7):2760 ADME Repeated doses CARCINOGENICITY Mode of action REPRODUCTIVE & DEVELOPMENTAL TOXICITY Endocrine Disruptor? SPECIALIZED STUDIE Immunotoxicity Neurotoxicity Endocrine activity Mode of Action Guidance for submission for food additive evaluations EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) - EFSA Journal 2012;10(7):2760