Expansion and Contraction Power P. Notes
advertisement

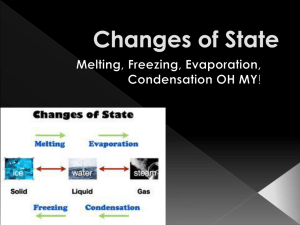
EXPANSION AND CONTRACTION KEY POINTS TO REMEMBER…. As the temperature of a solid, liquid or g as increases,the average distance between the atoms/molecules of the substance typically increases, causing the substance to expand. As the temperature of a solid, liquid or g as decreases, the average distance between the atoms/molecules typically decreases, causing the substance to contract. Heat expansion or contraction can happen to solids, liquids, and gases. Expansion or contraction due to changes in temperature is not permanent (e.g., objects that expand when heated can also contract when cooled). The number of atoms and the mass of the atoms DOES NOT CHANGE with changes in temperature. ONLY T HE SPACING BET WEEN PARTICLES CHANGES. Dif ferent types of solids, liquids or gases expand and contract dif ferently. For example solid metals that are made of pure elements like copper and aluminum are better at absorbing heat than alloy metals like brass. Gold is probably the best heat conductor, but it is so expensive that we don’t use it much. Brass is an alloy of copper and zinc. An alloy is a metal made from combining two or more metallic elements. EXPANSION AND CONTRACTION OF MATTER IN GENERAL Contraction As the average energy (temperature) of particles decrease, the space between the particles decrease. Matter in the solid liquid and gas phase CONTRACTS! Expansion As the average energy (temperature) of particles increase, the space between the particles increase. Matter in the solid liquid and gas phase EXPANDS! EXPANSION AND CONTRACTION IN SOLIDS Solids can EXPAND or CONTRACT depending on the temperature (average energy of the particles). For Example Metal Loop and Ball Demonstration Does the ball fit in the loop when the loop and ball are cool? Will the ball fit in the loop when the loop is heated? Why or Why Not? EXPANSION AND CONTRACTION IN LIQUIDS Liquids can EXPAND or CONTRACT depending on the temperature (average energy of the particles). This is demonstrated by the liquid used in a thermometer. As the liquid expands and contracts, it moves up and down the inside tubing ( the bore ) of the thermometer. Numbers are placed alongside the glass tube that mark the temperature when the line is at that point. This liquid is sometimes colored alcohol but can also be a metallic liquid called mercury. Both mercury and alcohol grow bigger when heated and smaller when cooled. GALILEO THERMOMETER A Galileo thermometer (or Galilean thermometer) is a thermometer made of a sealed glass cylinder containing a clearliquid and several glass vessels of varying densities. As temperature changes, the individual floats rise or fall in proportion to their respective density. The clear liquid in which the bulbs are submerged is not water, but some organic compound (such as ethanol) the density of which varies with temperature more than does water’s. This change of density of the outer clear liquid, with temperature change, causes the bulbs to rise or sink. GALILEO BAROMETER When a low -pressure weather system approaches, the weight of the air pressing on the open end of the spout decreases, allowing the water level to rise. Conversely, when a high pressure system moves in, it will push the water level down. A scale can be printed on the spout corresponding to the inches of mercury, normally between 28 and 32 inches. EXPANSION AND CONTRACTION IN GASES Gasses can EXPAND or CONTRACT depending on the temperature (average energy of the particles). What does Charles Gas Law say? (see book page 36) Under extremely high temperature conditions (like the temperatures inside the Sun, particles can be split into what makes them up (electrons and quarks etc). This creates a fourth state of matter called plasma. DOES VOLUME OR MASS CHANGE WHEN SOMETHING IS HEATED OR COOLED? Volume Before Heating Volume cm After Heating Volume cm Mass Balloon Only Balloon Tape Balloon, Tape and Air Air Only 2.16 g .01 g 2.31 g .14 g 2.16 g .01 g 2.31 g .14 g WHAT DO THE PARTICLES OF MATTER DO WHEN HEATED? Particles Particles EXAMPLES OF EXPANSION/CONTRACTION IN A SOLID, LIQUID AND GAS http://www.project2061.org/publications/EducatorsGuide/onl ine/Examples/Expansion/expansion.html STEEL BRIDGES Have expansion joints. BRIDGE EXPANSION JOINT EXAMPLE RAILROAD TRACKS Have gaps at each joint. RAILROAD RAILS CONCRETE ROADS Have expansion joints built in. CONCRETE ROADS CONCRETE ROADS SIDEWALKS Sidewalks are built with expansion joints to try and avoid the cracking that may occur. Sometimes a sidewalk will develop a crack even with expansion joints. Do you know what happens to the size of the cracks in the winter and summer? SIDEWALKS CARS Cars have engines with many working parts. The parts are engineered to be a little loose when cold so that when the engine becomes warm the parts do not expand to be to tight. STUDY THE CHART AND SUMMARIZE IN YOUR OWN WORDS IT’S MEANING. Quick Review ENGINEERING DISASTERS Tacoma Narrows Bridge QUIZ! Summary Sentences. 1. When matter is heated the particle’s speed _________ and the spacing_________. 2. When matter is cooled the particle’s speed _________ and the spacing _________.