P1 – Energy for the home
advertisement

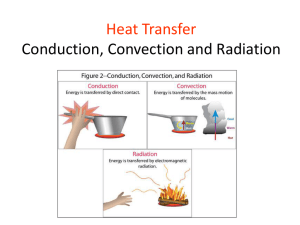
P1 – Energy for the home Revision lesson 1 Revision Lessons Does not contain all of the content for P1 – use checklists ..\P1 Energy for the home 2011\Summary and Checklists\P1 Checklist Foundation.doc ..\P1 Energy for the home 2011\Summary and Checklists\P1 Checklist Higher.doc 09/04/2015 Temperature and heat Temperature is a measurement of hotness. The temperature of an object is measured in degrees celsius (°C). Heat is a measurement of the energy of an object Heat energy is measured in Joules (J) Complete cool warm high higher low quicker thermograms cold warm Hot objects have a __________ temperature and usually __________ down. Cold objects have a __________temperature and usually __________ up. Energy will flow from a hotter object to a colder one. The greater the temperature difference, the faster the rate of cooling will be. For eg. A hot cup of coffee would cool down faster in a _______room than in a ______ room ____________________ are pictures in which colour is used to represent temperature. For warm objects, the __________ the temperature, the __________ they cool. Specific heat capacity The amount of energy needed to raise the temperature of something depends on: How much there is (mass) The temperature rise What it is (specific heat capacity) Energy = mass x specific heat capacity x temperature change Calculate the energy transferred when 80kg of water is heated from 10oC to 25oC. 80 x 4200 x (25-10) = 5 040 000 J SHC = how much energy something can store. The specific heat capacity of water is high therefore once heated it stores alot of energy, so its used for central heating Remember water has a higher SHC than most things so it can take more heat energy (time) to increase its temperature Specific latent heat Energy Mass x SLH Specific latent heat is the energy needed to change the state of 1kg of a substance without changing its temperature Specific latent heat water to stem = 2 260 000 j/kg Energy = mass x specific latent heat Calculate the energy transferred when 1.5kg of water in a kettle changes from liquid to a gas at 100oC. 1.5 x 2 260 000 = 3 390 000J Energy needed to change state, its different for all different materials Energy is put into break intermolecular bonds 150 This flat line shows where energy is being used to break the intermolecular bonds for evaporation 100 50 Melting point 0 solid heating -50 gas Boiling point liquid heating Time/s This flat line shows where energy is being used to break bonds – this has to be done during melting Heat transfer Heat can transfer in one of three ways: Conduction Convection Radiation Conduction Conduction is all about when heat is transferred through a _________. The heat is passed on by ___________ in the molecules. These vibrations get BIGGER when the solid has more ENERGY (i.e. when it is being __________). Heating a non-metal Heating a metal Metals are _______ conductors than non-metals. This is because the heat is carried by free ________ that can carry the energy around the metal and give it to other electrons and ions. Words – vibrations, electrons, solid, heated, better Conduction Cavity Walls How does a cavity wall prevent heat loss from a home? eat energy reaches the interior wall 1. H____ onducted through the wall 2. The heat energy is c_______ nsulator and 3. The air cavity between the two walls acts as an i_______ reduces heat loss by c_______ onduction . Double glazing How does double glazing keep a house warmer? Double glazing keeps a house warmer because there is a layer of ir between the panes of glass. a__ onductor so it acts Air is a poor c________, like an i_______, nsulator . educes heat loss The trapped air r_______ onduction from a house. by c_________ Side-view of double glazing 11 of 31 insulating layer of air © Boardworks Ltd 2005 Convection – Key Ideas Heat transfer through liquids and gases Heat causes particles in a fluid to: move apart → less dense → lighter → rises (and replace by cooler fluid) Convection Insert the Missing Words 2. On reaching the surface, cools when in the water _____ contact with the air 3. As the water cools it becomes more dense and sinks ______ heat 4. A cycle develops whereby warm water rises and cool water sinks, we call this a convection current __________ 1. As the water is heated it becomes ______ rises less dense and _____ Radiation – key facts All hot objects radiate heat. Called infra-red radiation Dull, dark surfaces are the best radiators and absorbers of heat Pale, shiny surfaces are the worst radiators and absorbers of heat Infra–red radiation can travel through a vacuum Radiation 09/04/2015 Radiation is when heat moves around in electromagnetic _________ like light does. Any hot object will emit heat radiation – the hotter it is, the more radiation it emits. This type of radiation is called __________, and too much of it will cause _________. Dark, matt colours will absorb AND emit the _____ infra-red radiation, and light, shiny colours will ________ it. The main difference with radiation is that conduction and convection could ONLY happen in solids, liquids or gases, whereas radiation will happen through an _____ _____. This is just as well, as otherwise we wouldn’t be able to get any heat from the ___. Words – sun, reflect, infra-red, waves, most, empty space, sunburn Anything HOT emits HEAT RADIATION – the hotter it is, the more infra red radiation it emits Thought shower: Where can heat be lost from your home?: Obviously, insulating your home can be quite expensive. Reduce energy loss from homes Roof - insulate loft; reduce conduction through ceiling and convection in loft space • Walls - cavity wall insulation; reduce conduction, convection, radiation (if shiny) Windows - double glazing; reduce conduction, convection if vacuum or gas at low pressure Doors - draught excluder; reduce conduction, convection Floors - carpets; trapped air pockets in wool are good insulators they reduce conduction Home insulation We can reduce the heat losses from our homes in many ways. Unscramble the list below to find out the ways: bouled zgnlagi vytiac llaw sunatoinil Drghaut xluecder fotl sunatoinil arcetps urctansi Why do each of these methods work? Describe which method of heat transfer they reduce Describe how energy is lost through the wall from the inside to the outside and how the insulation reduces the different types of energy loss. ! The quality of written communication will be assessed in your answer to this question. 6 marker question Energy losses in the home can be reduced by energy saving measures. One measure is to have double-glazed windows which reduce the amount of heat lost by conduction. The gap between the two pieces of glass is usually filled with a gas or a vacuum Describe how energy is lost through the wall from the inside to the outside and how the insulation reduces the different types of energy loss. ! The quality of written communication will be assessed in your answer to this question Payback and Efficiency Payback time is the time it takes for an insulation to pay for itself Payback time = Cost of improvement saving per year Efficiency = output e input useful energy out total energy in Payback and Efficiency questions Dan spends £120 on loft insulation. He is told that this will reduce his bill by £40 per year. Calculate the payback time. 2. What is payback time? 2. Dan heats his house with coal fires. He is told that his fires are 32% efficient. Explain what this means. 3. A kettle gives out 80J of heat to the room, and is supplied with 180 J of energy. How efficient is the kettle? 1. 120/40 = 3years 2. How long it will take the cost of the item to start saving on energy bills .Its the cost of insulation divided by annual saving. 3. How much useful energy is supplied from a device 4. 180-80 = useful = 100J 100/180 = 0.56 J 1. Plenary - To Do Make flash cards for: Heat and temperature Specific heat capacity and Specific latent heat Heat transfer (conduction, convection and radiation) Reducing energy losses from the home Payback and efficiency Now use them to test a partner