Specific heat
advertisement
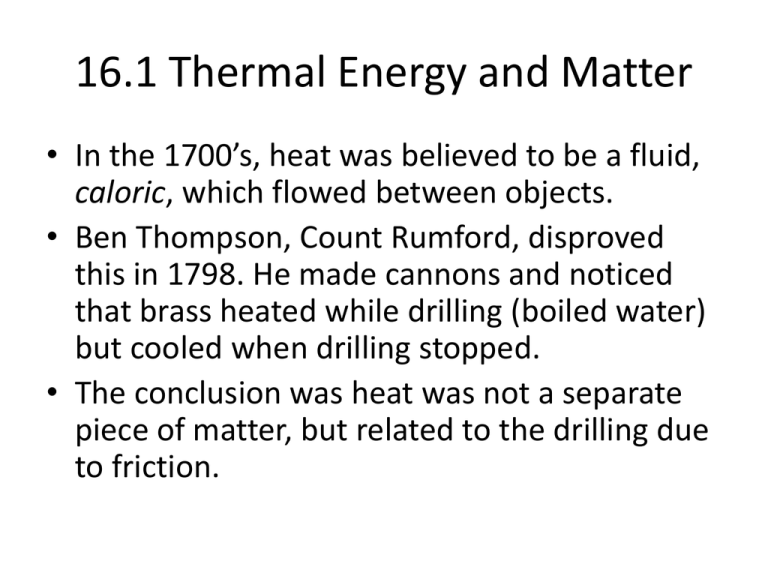
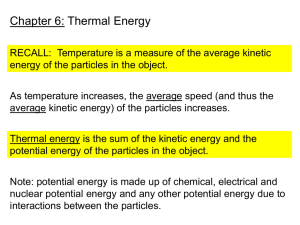
16.1 Thermal Energy and Matter • In the 1700’s, heat was believed to be a fluid, caloric, which flowed between objects. • Ben Thompson, Count Rumford, disproved this in 1798. He made cannons and noticed that brass heated while drilling (boiled water) but cooled when drilling stopped. • The conclusion was heat was not a separate piece of matter, but related to the drilling due to friction. Work and Heat • The transfer of thermal energy from one object to another because of a temperature difference is heat. • Heat flows spontaneously from hot objects to cold objects (“stealing” heat). Temperature • A measure of how hot or cold an object is compared to a reference point is temperature. • Absolute zero is 0 Kelvin. • Temperature is related to the average kinetic energy of the particles in an object due to their random motions through space. • Hot objects move faster, and heat is transferred by collisions. • High energy loses in collisions, and low energy gains in collisions (parked car that is hit moves). Thermal Energy • Thermal energy depends on the mass, temperature, and phase (solid, liquid, or gas) of an object. • Thermal energy is the total potential and kinetic energy of all the particles in an object. • Thermal energy depends on mass (longer to heat bucket of water than a droplet). Phases of Matter: Solid • Definite shape • Definite volume • slow-moving Phases of Matter: Liquid • • • • • Definite volume Indefinite shape Faster moving than solid Capillary action- can go against gravity Surface tension- the surface can hold (Red Rover) Phases of Matter: Gas • • • • Indefinite shape Indefinite volume Faster than liquid Moves erratically Thermal Contraction and Expansion • A balloon on a cold day will shrink, and tires deflate. This is due to the gas moving more slowly and exerting less force, or thermal contraction. • A balloon on a hot day could pop due to thermal expansion, or an increase in volume due to increase in temperature. • Thermal expansion occurs when particles of matter move farther apart as temperature increases. • Gases expand the most. • Thermometers depend on the expansion on alcohol (red fluid rises) or coil unwinding for a stove. Specific Heat • You do not get pizza burn from the crust, but the crust is the same temperature as the cheese that can burn the roof of your mouth. • This is because crust and cheese have different specific heats. • Specific heat is the amount of heat needed to raise the temperature of one gram of a material by one degree Celsius. • The lower a material’s specific heat, the more its temperature rises when a given amount of energy is absorbed by a given mass. • Water has a high specific heat of 4.18 J/gdegreeCelsius. Specific Heat • • • • • Q = mcT Q = heat (J) M = mass (g) C = specific heat (J/gdegree Celsius) T = change in temperature (degrees Celsius) Specific Heat problems • An iron skillet has a mass of 500 g. The specific heat of iron is 0.5 J/gdegreesCelsius. How much heat must be absorbed to raise the temperature 90 degrees Celsius? • Q = mcT • Q = (500)(0.5)(90) • Q = (250)(90) • Q = 22500 J, or 22.5 kJ Specific Heat Problems • How much heat would raise 100 g of water 100 degrees Celsius? • Q = mcT • Q = (100)(4.18)(100) • Q = (418)(100) • Q = 41800 J or 41.8 kJ Specific Heat Problems • How much heat is absorbed by 70 g of iron that goes from 25 degrees Celsius to 125 degrees Celsius? • Q = mcT • Q = (70)(0.5)(125-25) • Q = (70)(0.5)(100) • Q = (35)(100) • Q = 3500 J or 3.5 kJ Specific Heat Problems • An aquarium transfers 1200 kJ heat to 75,000 g of water. What is the increase in temperature? • Q = mcT • 1200000 = (75000)(4.18)T • 1200000 = 313500T • T = 4 degrees Celsius Specific Heat Problems • To release a diamond, a jeweler must heat a 10 g ring with 24 J of heat. What was the temperature change? • Q = mcT • 24 = (10)(0.2)T • 24 = 2T • T = 12 degrees Celsius Specific Heat Problems • What mass of water will change 3 degrees Celsius when 525 J of heat is added to it? • Q = mcT • 525 = (m)(4.18)(3) • 525 = (m)(12.54) • M = 42 g Measuring Heat Changes • A calorimeter is an instrument used to measure changes in thermal energy. • A calorimeter uses the principle that heat flows from a hotter object to a cooler object until both reach the same temperature. • Thermal energy of the burning food is absorbed by the surrounding water. • Water is stirred for even distribution of heat. • The calorimeter is made of an insulator.