Journal Club Pack - Circulation Research
advertisement

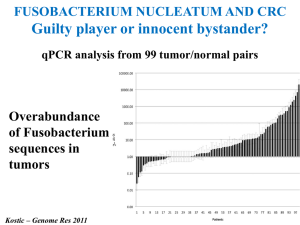
Circulation Research October 2012 Journal Club Gut Microbiota Metabolism of Anthocyanin Promotes Reverse Cholesterol Transport in Mice Via Repressing miRNA-10b Dongliang Wang, Min Xia, Xiao Yan, Dan Li, Lei Wang, Yuxuan Xu, Tianru Jin, Wenhua Ling Circ Res. 2012;111:967-981. PDF (with Online Supplement): http://circres.ahajournals.org/content/111/8/967.full.pdf+html Related Editorial by Stanley L. Hazen and Jonathan D. Smith [PDF]: An Antiatherosclerotic Signaling Cascade Involving Intestinal Microbiota, MicroRNA-10b, and ABCA1/ABCG1-Mediated Reverse Cholesterol Transport Included in the Journal Club pack: Abstract, Novelty & Significance section, and all figures. Gut Microbiota Metabolism of Anthocyanin Promotes Reverse Cholesterol Transport in Mice Via Repressing miRNA-10b Abstract Rationale: We and others have demonstrated that anthocyanins have antiatherogenic capability. Because intact anthocyanins are absorbed very poorly, the low level of circulating parent anthocyanins may not fully account for their beneficial effect. We found recently that protocatechuic acid (PCA), a metabolite of cyanidin-3 to 0-β-glucoside (Cy-3-G), has a remarkable antiatherogenic effect. Objective: To investigate whether mouse gut microbiota metabolizes Cy-3-G into PCA and to determine whether and how PCA contributes to the antiatherogenic potency of its precursor, Cy-3-G. Methods and Results: PCA was determined as a gut microbiota metabolite of Cy-3-G in ApoE−/− mice, verified by the utilization of antibiotics to eliminate gut microbiota and further microbiota acquisition. PCA but not Cy-3-G at physiologically reachable concentrations promoted cholesterol efflux from macrophages and macrophage ABCA1 and ABCG1 expression. By conducting a miRNA microarray screening, we revealed that expression of miRNA-10b in macrophages can be reduced by PCA. Functional analyses demonstrated that miRNA-10b directly represses ABCA1 and ABCG1 and negatively regulates cholesterol efflux from murine- and human-derived macrophages. Further in vitro and ex vivo analyses verified that PCA accelerates macrophage cholesterol efflux, correlating with the regulation of miRNA-10bABCA1/ABCG1 cascade, whereas Cy-3-G consumption promoted macrophage RCT and regressed atherosclerotic lesion in a gut microbiotaendependent manner. Conclusions: PCA, as the gut microbiota metabolite of Cy-3-G, exerts the antiatherogenic effect partially through this newly defined miRNA-10b-ABCA1/ABCG1-cholesterol efflux signaling cascade. Thus, gut microbiota is a potential novel target for atherosclerosis prevention and treatment. Novelty and Significance What Is Known? •Anthocyanins, similar to other flavonoids, have been generally recognized as cardioprotective compounds despite their very limited bioavailability. •Protocatechuic acid (PCA), one metabolite of cyanidin-3-O-β-glucoside (Cy-3-G), has been shown to inhibit the development of early atherosclerosis. •miRNAs are known to regulate macrophage cholesterol efflux via altering expression of their target genes ATP-binding cassette transporter A1 (ABCA1) and G1 (ABCG1), both of which facilitate cellular cholesterol efflux. What New Information Does This Article Contribute? •PCA, a gut microbiota metabolite of Cy-3-G, mediates the stimulatory effects of its precursor Cy-3-G on macrophage reverse cholesterol transport (RCT) and established atherosclerosis regression. •miRNA-10b negatively regulates macrophage cholesterol efflux via altering its target genes ABCA1 and ABCG1 expression, whereas PCA reduces miRNA-10b levels, the net effect being enhanced macrophage cholesterol efflux and atherosclerosis regression. The relatively low concentrations of intact anthocyanins in vivo have cast doubt about their antiatherogenic effect. Because anthocyanins, similar to other flavonoids, are intensively metabolized by gut microbiota, we tested the protective effects of PCA on atherosclerosis. We showed that the stimulatory effects of anthocyanin Cy-3-G on macrophage RCT and established atherosclerosis regression were mediated by PCA. We also showed that miRNA-10b negatively regulates cholesterol efflux from murine- and humanderived macrophages by repressing expression of ABCA1 and ABCG1, whereas PCA reduces miR-10b levels, which together contribute to accelerated cholesterol efflux from macrophages and established atherosclerosis regression. The discovery of a relationship between gut microbiotaendependent metabolism of dietary anthocyanin and regression of atherosclerosis provides for the opportunity for the utilization of gut microbiota in the treatment of atherosclerosis. Moreover, our novel findings also support the notion that miRNA-10b might be an attractive candidate for promotion macrophage RCT and established atherosclerosis regression. PCA is a gut microbiota metabolite of Cy-3-G. Wang D et al. Circulation Research 2012;111:967-981 Copyright © American Heart Association PCA but not Cy-3-G promotes cholesterol efflux from murine- and human-derived macrophages. Wang D et al. Circulation Research 2012;111:967-981 Copyright © American Heart Association ABCA1 and ABCG1 genes are direct targets of miR-10b. Wang D et al. Circulation Research 2012;111:967-981 Copyright © American Heart Association miR-10b regulates the ability of macrophages to efflux free cholesterol. Wang D et al. Circulation Research 2012;111:967-981 Copyright © American Heart Association miR-10b regulates the stimulatory effect of PCA on cholesterol efflux in macrophages. Wang D et al. Circulation Research 2012;111:967-981 Copyright © American Heart Association Cy-3-G accelerates cholesterol efflux from macrophages ex vivo and in vivo through its metabolite PCA. Male 30-week-old ApoE−/− mice with or without the antibiotics were orally gavaged with Cy-3-G (50 mg/kg BW), PCA (5 mg/kg BW), Cy-3-G (50 mg/kg BW) plus P... Wang D et al. Circulation Research 2012;111:967-981 Copyright © American Heart Association Cy-3-G regresses endogenous foam cell formation and establishes atherosclerosis through its metabolite PCA in vivo. Wang D et al. Circulation Research 2012;111:967-981 Copyright © American Heart Association