Evaporation - Civil & Environmental Engineering
advertisement
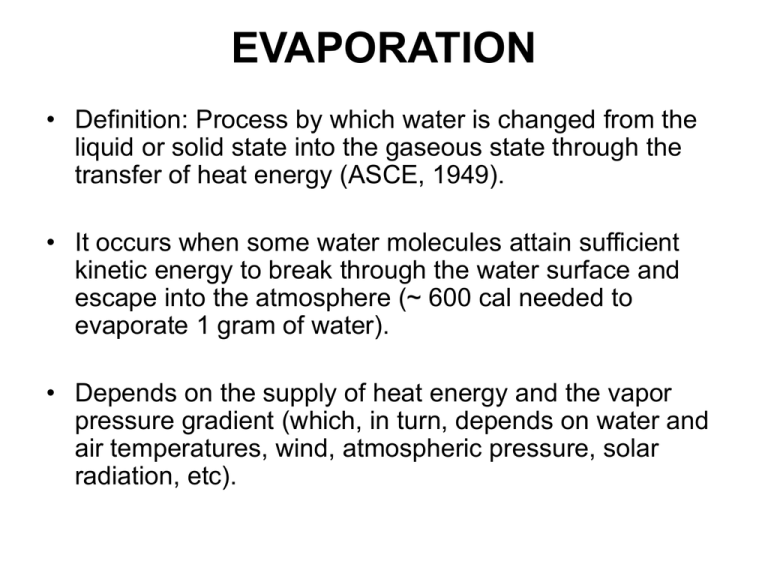
EVAPORATION • Definition: Process by which water is changed from the liquid or solid state into the gaseous state through the transfer of heat energy (ASCE, 1949). • It occurs when some water molecules attain sufficient kinetic energy to break through the water surface and escape into the atmosphere (~ 600 cal needed to evaporate 1 gram of water). • Depends on the supply of heat energy and the vapor pressure gradient (which, in turn, depends on water and air temperatures, wind, atmospheric pressure, solar radiation, etc). TRANSPIRATION (T) • Transpiration is the evaporation occurring through plant leaves (stomatal openings). • Transpiration is affected by plant physiology and environmental factors, such as: - Type of vegetation - Stage and growth of plants - Soil conditions (type and moisture) - Climate and weather EVAPOTRANSPIRATION (ET) • Combined “loss” of water vapor from within the leaves of plants (“transpiration”) and evaporation of liquid water from water surfaces, bare soil and vegetative surfaces. • Globally, about 62% of the precipitation that falls on the continent is evapotranspired (~72,000 km3/yr); 92% of which from land surfaces evapotranspiration and 3% from open water evaporation (source: Dingman, “Physical Hydrology”). • Approximately 70% of the mean annual rainfall in the U.S. is returned to the atmosphere as evaporation or transpiration. EVAPOTRANSPIRATION (ET) • In practice, the terms E and ET are often used to mean the same thing - the evaporation from the land surface. • Therefore, you must use the context to determine what the term evaporation means in a specific case (i.e., is it just from an open water surface or the entire land surface?). POTENTIAL EVAPORATION (PE) • is the climate controlled evaporation from an open water surface with unlimited supply (and no thermal capacity). POTENTIAL EVAPOTRANSPIRATION (PET) • is the ET that would occur from a well vegetated surface when moisture supply is not limiting (often calculated as the PE). • Actual evapotranspiration (AET; ET) drops below its potential level as the soil dries. DESIGN • Evaporation must be considered in the design of large water storage reservoirs, large-scale water resources planning and water supply studies. • For flood flow studies, urban drainage design applications it may be neglected. • Example: during typical storm periods with intensities of 0.5 in/hr, evaporation is on the order of 0.01 in/hr. METHODS FOR ESTIMATING EVAPORATION • Water budget methods • Energy budget methods • Mass transfer techniques (e.g., Meyer, Thornthwaile-Holzman) • Combination of energy budget and mass transfer methods (e.g.,Penman) Energy budget method Total solar radiation - Rt Net energy advected (net energy content of incoming and outcoming water - Ee Reflected solar radiation - Rr Energy used for evaporation (latent heat)- Ee Sensible heat loss from the water body to the atmosphere - Hn Net long-wave radiation exchange between the atmospere and the water body- R1 Energy stored - Es R1 includes long-wave (LW) radiation from the atmosphere, reflected LW radiation, LW radiation emitted by water Energy budget method g cal Es E a R t R r E e H n R1 2 cm - day Ee Rt Rr Ee Hn R1 Es R1 includes long-wave (LW) radiation from the atmosphere, reflected LW radiation, LW radiation emitted by water Energy budget method • Amount of evaporation - E mm Ee E 10 Hv day g cal Hv 596 0 . 52 T - latent heat of vaporizat ion 3 cm T C - temperatu re of the water surface Energy budget method Characteristics: • most accurate method (evaporation is a function of the energy state of the water system) • difficult to evaluate all terms • energy balance equation has to be simplified • empirical formulas are used (although radiation measurements are preferable) Water budget method S t P Q Q r Q s Q 0 Q d E E Precipitation - P Evaporation- E Inflow- Q Surface runoff - Qr Subsurface runoff - Qs Outflow- Q0 Subsurface seepage losses- Qd Water budget method • Units: ac - ft E month • Depth of evaporation: in 12 E E day nA p * mm 12 ( 25 . 4 ) E E nA p day * n – number of days Ap – area of the pond [ac] Water budget method Characteristics: - Simple - Difficult to estimate Qd and Qs - Unreliable, accuracy will increase as Δt increases • Example on water balance model Mass transfer methods - definitions 17 . 3T e s [ mb ] 6 . 11 exp ; T 237 . 3 e s mm Hg Rh e s [ mb ] ; Table 14.1 1 . 36 e es e – actual vapor pressure (difference in the atmospheric pressure with and without the vapor) es – saturated vapor pressure (partial pressure of water vapor in saturated air) T [ºC] – air temperature Rh – relative humidity • Evaporation is a diffusive process (moves from where its concentration is larger to where its concentration is smaller at a rate that is proportional to the gradient of concentration): E = b0 (es0 – ea) es0 – vapore pressure of the evaporating surface; saturation vapor pressure at the water surface temperature Ts - ea – vapor pressure of overlying air at the same height - b0 – empirical coefficient that has to be calibrated • E = b0 (es0 – ea) • Studies showed that b0 = function (air turbulence)=fn(v) • E = b1 fn(v)(es – ea) • Meyer’s formula: E = 0.5 (1 + 0.1 v30)(es – ea) v30 - wind speed [mi/h] at 30 ft height; es; ea [in Hg] E [in/day] • b0 = f(v, es, ea, Ta, Tw) • Thornthwaite-Holzman equation (no calibration) b0 = f(v,T,k); k – Von Karman constant (0.41) 833 k ( e1 e 2 )( v 2 v1 ) 2 E 2 z2 T 459 . 4 ln z1 • Example Combination approach – Penman equation • Combine mass-transfer and energy-balance equations to derive an evaporation equation that does not require water surface temperature data. (14 24 ) Hw E n E ao Hw mm - evaporatio n day En mm - net radiation day E ao mm - mass transfer day Hw Penman equation: (14 14 ) e0 e * a T0 T 1 E n E ao 25 , 083 1 . 36 T 237 . 3 2 17 . 3T exp T 237 . 3 Δ [mm Hg/ºC] – slope of the saturation vapor pressure curve at mean temperature T0 [ºC] – temperature of the water surface T [ºC] – temperature of the air e0 [mm Hg] - vapor pressure of the water surface ea* [mm Hg] - saturated vapor pressure at temperature T Penman equation: Hw E n E ao • En [mm/day] – net radiation g - cal • Start with energy equation: R n RI R B 2 cm day Rn – net radiation RI – amount of energy absorbed (shortwave) RB – net outward flow of longwave radiation Penman equation: Hw E n E ao Rn RI RB n R I R A 1 r a b D RI [g-cal/cm2-day] – amount of energy absorbed (shortwave) RA [g-cal/cm2-day] – total possible radiation for the period of estimation; it is function of latitude and season; Table 14-3. r – reflection coef. (0.05-0.12) a,b – empirical coef. (a=0.2; b=0.5) n/D – fraction of possible sunshine (from climatic atlas) Rn RI RB R B T 273 0 . 47 0 . 077 4 1 . 1777 10 7 n e 0 .2 0 .8 D cal 2 4 cm C day • Rn[g-cal/cm2-day] – net radiation • RI [g-cal/cm2-day] – amount of energy absorbed (shortwave) • RB [g-cal/cm2-day] – net outward flow of longwave radiation • e [mm Hg] – actual vapor pressure • T [ºC] – air temperature • n/D – fraction of possible sunshine (from climatic atlas) Penman equation: mm Rn En 10 Hv day Hw E n E ao net radiation g - cal Rn net radiation 2 cm day g - cal Hv 596 0 . 52 T - latent heat of vaporizat ion 3 cm Penman equation: Hw E n E ao g - cal Rn 2 cm day mm Rn En 10 Hv day g - cal Hv 596 0 . 52 T 3 cm • En – net radiation • Rn – net radiation •Hv –latent heat of vaporization Penman equation: c p pa Hw E n E ao (14 - 11) 0 . 62 H v c p specific heat of air at constant p a atmospheri pressure c pressure g - cal Hv 596 0 . 52 T - latent heat of vaporizat ion 3 cm 0.485 (typical value) H w (E ) Penman equation: E ao mm - mass transfer day E n E ao (based on diffusivit E a 0 0 . 35 e s e 0 . 2 0 . 55 V e s mm Hg - saturated y law) vapor pressure at air temper ature T a e mm Hg - actual vapor pressure at air temper ature T a V [m/sec] - wind spead at 2 m height Penman equation: mm E n E ao Hw day - evaporatio n E acre - ft - total evaporatio n mm 1 1 E acre - ft H w n days Area acres 12 25 . 4 day example Measuring evaporation Irrigated lysimeter PE = Rainfall + Irrigation - Percolation Atmometer U.S. Weather Bureau Class A Pan 4 ft Wooden support 10 in 6 in Galvanized steel Evaporation pan S t P Q Q r Q s Q 0 Q d E P Ep Surface runoff - Qr S t Ep P S t Evaporation - E Precipitation - P Inflow- Q Subsurface runoff - Qs Outflow- Q Subsurface seepage losses- Qd Evaporation Pan • Historical records of daily pan evaporation are available from the National Climatic Data Center (NCDC) for U.S. Weather Buruau Class A Land pans. Evaporation Pan • We are not really interested in what evaporates from a pan; instead we want to know the regional evaporation from land surface or the evaporation from a nearby lake. Unfortunately, pan evaporation is often a poor indicator of these variables (due in part to pan boundary effects and limited heat storage). Evaporation Pan • Evaporation from an open water surface (E) is usually estimated from the pan evaporation (Ep) as: E = K Ep where K is the pan coefficient (regional coef, usually around ~0.7). Similar expressions are also used in practice to estimate potential evapotranspiration from pan data. Pan coefficient FIGURE 2. Source: Farnsworth, Richard K., Edwin S. Thompson, and Eugene L. Peck. After Map 4: Pan Coefficients. In NOAA Technical Report NWS 33, Evaporation Atlas for the Contiguous 48 United States, NWS, NOAA, 1982. evapotranspiration from satellite data • When a surface evaporates, it looses energy and cools itself. It is that cooling that can be observed from space. Satellites can map the infrared heat radiated from Earth, thus enabling to distinguish the cool surfaces from the warm surfaces. winter summer