Selivanoffs - mStudyGroup.com
advertisement

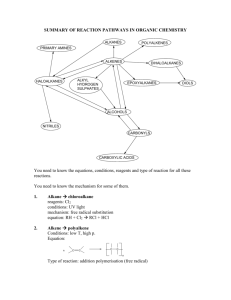
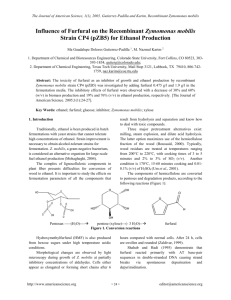
Selivanoff’s Test To distinguish between ketose and Aldose sugar Selivanoff’s test is positive only for ketose sugars and so is answered by Fructose, sucrose and other fructose containing carbohydrates. Selivanoff’s reagent consists of: Resorcinol Concentrated Hydrochloric acid Selivanoff’s reagent is prepared by dissolving 0.05g resorcinol in 50ml of distilled water. Add 33ml of concentrated hydrochloric acid (36%) in it very slowly. Add distilled water to make the total volume 100ml. In Selivanoff’s reagent, the final concentration of hydrochloric acid will be about 12% as the strength of concentrated hydrochloric acid is 36% Volume of acid/Volume of solution*Concentration of acid =33ml/100ml *36%=12% The carbohydrates are converted into furfural derivatives by the concentrated HCL present in the Selivanoff’s reagent. Only furfural derivatives of ketohexose (5-hydroxymethyl furfural) condense with resorcinol to form cherry red colored complex. fructose+HCl Hydroxymethyl furfural + Levulinic acid Hydroxymethyl furfural + Resorcinol Cherry red colored complex Sucrose will also give Selivanoff’s test positive because the acidity of reagent is sufficient enough to hydrolyze sucrose to glucose and fructose. When sugars are treated with strong acids, on heating, they are hydrolyzed into monosaccharide. These monosaccharide undergo dehydration(removal of water molecule) by the acids. Depending on the type of monosaccharide these dehydrated monosaccharide are called furfural or furfural derivatives. C5H10O5 -3H2O C5H4O2 pentose C6H12O6 Hexose -3H2O Furfural C6H6O3 Hydroxymethyl furfural Take 3ml of Selivanoff’s reagent and 1ml of the given carbohydrate solution in a test tube and mix them. Boil for 30 seconds only and then cool the solution. Note the appearance of colour. The appearance of a cherry red or pink colour within 30 seconds indicates the presence of a ketohexose. The final concentration of hydrochloric acid in the Selivanoff’s reagent should not exceed 12%. In the presence of excessive strong acid, aldohexoses may be converted into ketohexoses and thus it will give a positive test. Prolonged boiling may also convert the aldohexoses into ketohexoses. Hence boiling should be restricted to 30 seconds. Experiment Observation Inference 3ml of Selivanoff’s reagent + 1ml of the given solution mix them and Boil for 30 sec. and cool the solution. cherry red or pink colour in case of a ketohexose. Ketose sugar is present