Studying Stress and Acute Toxicity Using
advertisement
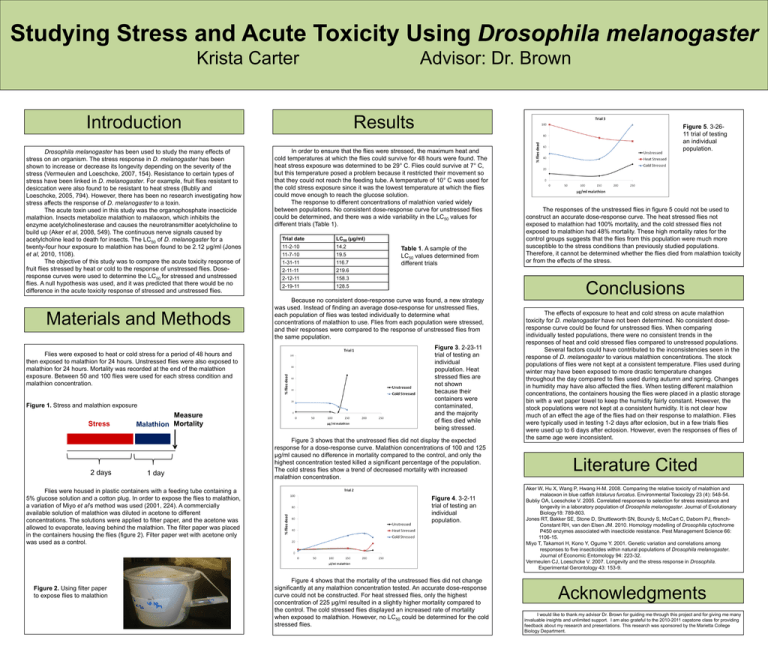
Studying Stress and Acute Toxicity Using Drosophila melanogaster Krista Carter Advisor: Dr. Brown Introduction Drosophila melanogaster has been used to study the many effects of stress on an organism. The stress response in D. melanogaster has been shown to increase or decrease its longevity depending on the severity of the stress (Vermeulen and Loeschcke, 2007, 154). Resistance to certain types of stress have been linked in D. melanogaster. For example, fruit flies resistant to desiccation were also found to be resistant to heat stress (Bubliy and Loeschcke, 2005, 794). However, there has been no research investigating how stress affects the response of D. melanogaster to a toxin. The acute toxin used in this study was the organophosphate insecticide malathion. Insects metabolize malathion to malaoxon, which inhibits the enzyme acetylcholinesterase and causes the neurotransmitter acetylcholine to build up (Aker et al, 2008, 549). The continuous nerve signals caused by acetylcholine lead to death for insects. The LC50 of D. melanogaster for a twenty-four hour exposure to malathion has been found to be 2.12 µg/ml (Jones et al, 2010, 1108). The objective of this study was to compare the acute toxicity response of fruit flies stressed by heat or cold to the response of unstressed flies. Doseresponse curves were used to determine the LC50 for stressed and unstressed flies. A null hypothesis was used, and it was predicted that there would be no difference in the acute toxicity response of stressed and unstressed flies. Materials and Methods Flies were exposed to heat or cold stress for a period of 48 hours and then exposed to malathion for 24 hours. Unstressed flies were also exposed to malathion for 24 hours. Mortality was recorded at the end of the malathion exposure. Between 50 and 100 flies were used for each stress condition and malathion concentration. Figure 1. Stress and malathion exposure Stress 2 days Measure Malathion Mortality 1 day Flies were housed in plastic containers with a feeding tube containing a 5% glucose solution and a cotton plug. In order to expose the flies to malathion, a variation of Miyo et al’s method was used (2001, 224). A commercially available solution of malathion was diluted in acetone to different concentrations. The solutions were applied to filter paper, and the acetone was allowed to evaporate, leaving behind the malathion. The filter paper was placed in the containers housing the flies (figure 2). Filter paper wet with acetone only was used as a control. Figure 2. Using filter paper to expose flies to malathion Results In order to ensure that the flies were stressed, the maximum heat and cold temperatures at which the flies could survive for 48 hours were found. The heat stress exposure was determined to be 29° C. Flies could survive at 7° C, but this temperature posed a problem because it restricted their movement so that they could not reach the feeding tube. A temperature of 10° C was used for the cold stress exposure since it was the lowest temperature at which the flies could move enough to reach the glucose solution. The response to different concentrations of malathion varied widely between populations. No consistent dose-response curve for unstressed flies could be determined, and there was a wide variability in the LC50 values for different trials (Table 1). Trial date LC50 (µg/ml) 11-2-10 14.2 11-7-10 19.5 1-31-11 116.7 2-11-11 219.6 2-12-11 158.3 2-19-11 128.5 Table 1. A sample of the LC50 values determined from different trials Because no consistent dose-response curve was found, a new strategy was used. Instead of finding an average dose-response for unstressed flies, each population of flies was tested individually to determine what concentrations of malathion to use. Flies from each population were stressed, and their responses were compared to the response of unstressed flies from the same population. Figure 3. 2-23-11 trial of testing an individual population. Heat stressed flies are not shown because their containers were contaminated, and the majority of flies died while being stressed. Figure 3 shows that the unstressed flies did not display the expected response for a dose-response curve. Malathion concentrations of 100 and 125 µg/ml caused no difference in mortality compared to the control, and only the highest concentration tested killed a significant percentage of the population. The cold stress flies show a trend of decreased mortality with increased malathion concentration. Figure 4. 3-2-11 trial of testing an individual population. Figure 4 shows that the mortality of the unstressed flies did not change significantly at any malathion concentration tested. An accurate dose-response curve could not be constructed. For heat stressed flies, only the highest concentration of 225 µg/ml resulted in a slightly higher mortality compared to the control. The cold stressed flies displayed an increased rate of mortality when exposed to malathion. However, no LC50 could be determined for the cold stressed flies. Figure 5. 3-2611 trial of testing an individual population. The responses of the unstressed flies in figure 5 could not be used to construct an accurate dose-response curve. The heat stressed flies not exposed to malathion had 100% mortality, and the cold stressed flies not exposed to malathion had 48% mortality. These high mortality rates for the control groups suggests that the flies from this population were much more susceptible to the stress conditions than previously studied populations. Therefore, it cannot be determined whether the flies died from malathion toxicity or from the effects of the stress. Conclusions The effects of exposure to heat and cold stress on acute malathion toxicity for D. melanogaster have not been determined. No consistent doseresponse curve could be found for unstressed flies. When comparing individually tested populations, there were no consistent trends in the responses of heat and cold stressed flies compared to unstressed populations. Several factors could have contributed to the inconsistencies seen in the response of D. melanogaster to various malathion concentrations. The stock populations of flies were not kept at a consistent temperature. Flies used during winter may have been exposed to more drastic temperature changes throughout the day compared to flies used during autumn and spring. Changes in humidity may have also affected the flies. When testing different malathion concentrations, the containers housing the flies were placed in a plastic storage bin with a wet paper towel to keep the humidity fairly constant. However, the stock populations were not kept at a consistent humidity. It is not clear how much of an effect the age of the flies had on their response to malathion. Flies were typically used in testing 1-2 days after eclosion, but in a few trials flies were used up to 6 days after eclosion. However, even the responses of flies of the same age were inconsistent. Literature Cited Aker W, Hu X, Wang P, Hwang H-M. 2008. Comparing the relative toxicity of malathion and malaoxon in blue catfish Ictalurus furcatus. Environmental Toxicology 23 (4): 548-54. Bubliy OA, Loeschcke V. 2005. Correlated responses to selection for stress resistance and longevity in a laboratory population of Drosophila melanogaster. Journal of Evolutionary Biology18: 789-803. Jones RT, Bakker SE, Stone D, Shuttleworth SN, Boundy S, McCart C, Daborn PJ, ffrenchConstant RH, van den Elsen JM. 2010. Homology modelling of Drosophila cytochrome P450 enzymes associated with insecticide resistance. Pest Management Science 66: 1106-15. Miyo T, Takamori H, Kono Y, Ogume Y. 2001. Genetic variation and correlations among responses to five insecticides within natural populations of Drosophila melanogaster. Journal of Economic Entomology 94: 223-32. Vermeulen CJ, Loeschcke V. 2007. Longevity and the stress response in Drosophila. Experimental Gerontology 43: 153-9. Acknowledgments I would like to thank my advisor Dr. Brown for guiding me through this project and for giving me many invaluable insights and unlimited support. I am also grateful to the 2010-2011 capstone class for providing feedback about my research and presentations. This research was sponsored by the Marietta College Biology Department.