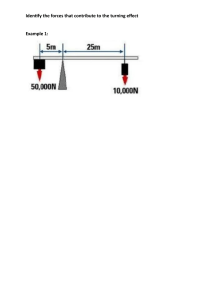
Faculty of Science Department of Chemistry Academic Year 2022-2023 – Term-III Grade 9 Am – Test 1 3.7 Chemical Reactions and Chemical Equations Name: _____________________ Date: ______________ Section: __________ Indicate the number of atoms for each of the elements in the chemical equations below and then decide if the equation is balanced or not balanced Example: H2 + O2 H2O Elements Reactants side Product side Equal masses? H 2 2 Yes O 2 1 No Is the equation balanced or not balanced? __No__ N2 + 3H2 Elements Reactants side 2NH3 Product side N H Is the equation balanced or not balanced? __________ Equal masses? Faculty of Science Department of Chemistry Academic Year 2022-2023 – Term-III Grade 9 Am – Test 1 3.7 Chemical Reactions and Chemical Equations 2KClO3 Elements Reactants side KCl + 3O2 Equal masses? Product side K Cl O Is the equation balanced or not balanced? __________ 2NaCl + F2 Elements Reactants side 2NaF + Cl2 Product side Na Cl F Is the equation balanced or not balanced? __________ Equal masses?