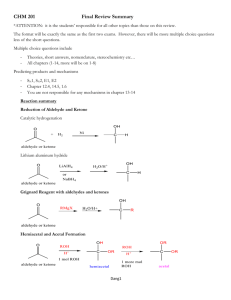
Powered by AI Copilot Certainly! Let’s delve into the fascinating world of hemiacetals and hemiketals. These compounds play a crucial role in biochemistry and carbohydrate chemistry. 🧪🔬 1. Hemiacetals: When an alcohol adds to an aldehyde, the resulting product is called a hemiacetal. The prefix “hemi” (meaning “half”) is used because, as we’ll see, a second alcohol can further react, leading to species called acetals. The conversion of an alcohol and an aldehyde to a hemiacetal is a reversible process. In the context of glucose and other sugars, five- and six-membered cyclic hemiacetals are particularly stable and favored at equilibrium due to the inherent stability of these ring sizes . Biochemical mechanism of hemiacetal formation: At physiological pH, hemiacetals (and hemiketals) are higher in energy than their aldehydealcohol components, so the equilibrium lies to the left. However, cyclic hemiacetals are considerably lower in energy and are favored at equilibrium . In summary, hemiacetals are essential players in sugar chemistry! 2. Hemiketals: When an alcohol adds to a ketone, the resulting product is a hemiketal. Similar to hemiacetals, hemiketals can also form cyclic structures. Glucose (an aldose) exists as a cyclic hemiacetal, while fructose (a ketose) exists as a cyclic hemiketal . 3. Formation Mechanism: In the organic laboratory, hemiacetal and hemiketal formation typically occurs in the presence of a strong acid. The acid catalyzes the reaction by protonating the carbonyl oxygen, increasing the electrophilicity of the carbonyl carbon. The overall process involves protonation, nucleophilic attack by the alcohol, deprotonation, and removal of water . Remember, these reactions are fundamental in understanding the behavior of carbohydrates and other organic compounds. Chemistry is full of delightful surprises! 🌟🔍