answers-to-end-of-chapter -combined and coordinated -textbook-questionspdf-pdf-free
advertisement
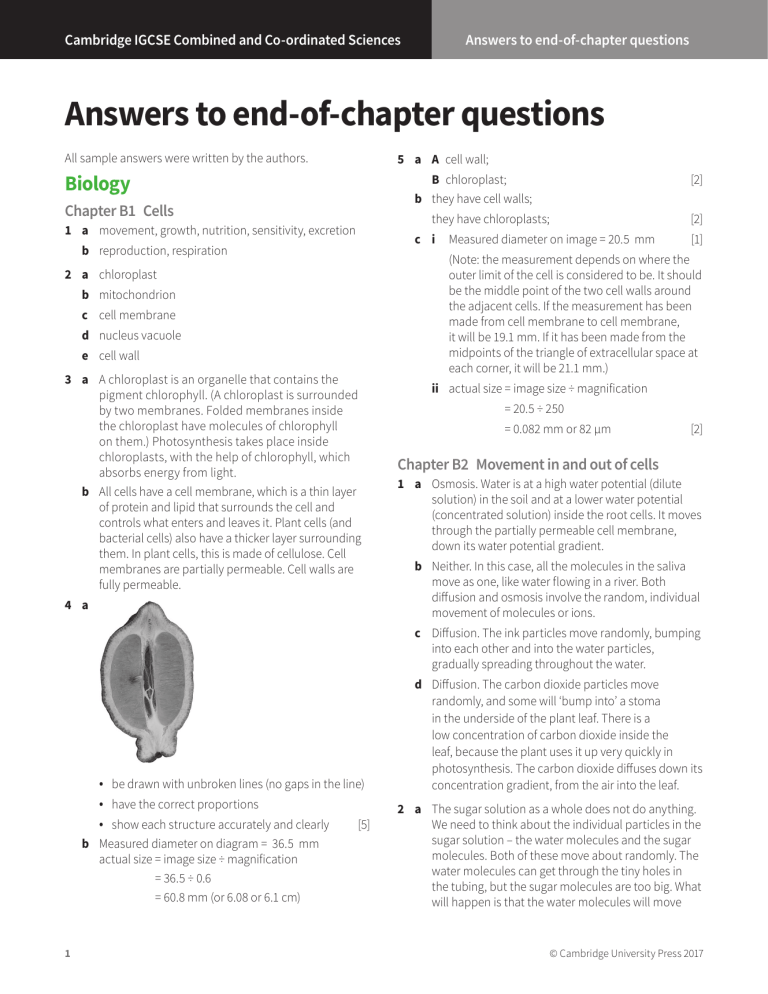
Cambridge IGCSE Combined and Co-ordinated Sciences Answers to end-of-chapter questions Answers to end-of-chapter questions All sample answers were written by the authors. Biology Chapter B1 Cells 1 a movement, growth, nutrition, sensitivity, excretion b reproduction, respiration 2 a chloroplast b mitochondrion c cell membrane d nucleus vacuole e cell wall 3 aA chloroplast is an organelle that contains the pigment chlorophyll. (A chloroplast is surrounded by two membranes. Folded membranes inside the chloroplast have molecules of chlorophyll on them.) Photosynthesis takes place inside chloroplasts, with the help of chlorophyll, which absorbs energy from light. b All cells have a cell membrane, which is a thin layer of protein and lipid that surrounds the cell and controls what enters and leaves it. Plant cells (and bacterial cells) also have a thicker layer surrounding them. In plant cells, this is made of cellulose. Cell membranes are partially permeable. Cell walls are fully permeable. 4 a 5 a A cell wall; B chloroplast; b they have cell walls; [2] they have chloroplasts; c i Measured diameter on image = 20.5 mm [2] [1] (Note: the measurement depends on where the outer limit of the cell is considered to be. It should be the middle point of the two cell walls around the adjacent cells. If the measurement has been made from cell membrane to cell membrane, it will be 19.1 mm. If it has been made from the midpoints of the triangle of extracellular space at each corner, it will be 21.1 mm.) ii actual size = image size ÷ magnification = 20.5 ÷ 250 = 0.082 mm or 82 µm [2] Chapter B2 Movement in and out of cells 1 aOsmosis. Water is at a high water potential (dilute solution) in the soil and at a lower water potential (concentrated solution) inside the root cells. It moves through the partially permeable cell membrane, down its water potential gradient. bNeither. In this case, all the molecules in the saliva move as one, like water flowing in a river. Both diffusion and osmosis involve the random, individual movement of molecules or ions. cDiffusion. The ink particles move randomly, bumping into each other and into the water particles, gradually spreading throughout the water. • be drawn with unbroken lines (no gaps in the line) • have the correct proportions • show each structure accurately and clearly [5] b Measured diameter on diagram = 36.5 mm actual size = image size ÷ magnification = 36.5 ÷ 0.6 = 60.8 mm (or 6.08 or 6.1 cm) 1 dDiffusion. The carbon dioxide particles move randomly, and some will ‘bump into’ a stoma in the underside of the plant leaf. There is a low concentration of carbon dioxide inside the leaf, because the plant uses it up very quickly in photosynthesis. The carbon dioxide diffuses down its concentration gradient, from the air into the leaf. 2 aThe sugar solution as a whole does not do anything. We need to think about the individual particles in the sugar solution – the water molecules and the sugar molecules. Both of these move about randomly. The water molecules can get through the tiny holes in the tubing, but the sugar molecules are too big. What will happen is that the water molecules will move © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences If Visking tubing containing a sugar solution is put into a beaker of water, water will move from the water into the sugar solution, by osmosis. b It is true that plant cells do not burst in pure water, but this is because the cell wall is strong enough to prevent this happening. The cell wall is fully permeable, and cannot stop water molecules going through it. So the corrected sentence could be: Plant cells do not burst in pure water because, although water enters the cell by osmosis, the strong wall prevents the cell from bursting. c It is true that water will move out of a plant cell by osmosis, if the cell is placed in a concentrated sugar solution. However, the cell wall is not partially permeable – it is fully permeable. So the corrected sentence could be: When a plant cell is placed in a concentrated sugar solution, water moves out of the cell by osmosis, through the partially permeable cell membrane. d Plasmolysis is the result of placing a plant cell in a concentrated sugar solution. So much water moves out of the cell by osmosis that the contents shrink, and the cell membrane pulls away from the cell wall. As animal cells do not have a cell wall, they cannot undergo plasmolysis. So the corrected sentence could be either: Animal cells shrink when placed in a concentrated sugar solution. OR Plant cells plasmolyse in a concentrated sugar solution. 3 aDiffusion is a result of the random movement of molecules or ions. At higher temperatures, these have more kinetic energy and move faster, so diffusion happens faster. b During daylight, plants photosynthesise. They produce oxygen in their leaves, so the oxygen concentration inside the leaf is higher than the oxygen concentration in the air outside. Oxygen therefore diffuses down its concentration gradient, from the leaf and into the air. c Visking tubing is a partially permeable membrane. It has tiny, molecule-sized holes in it. Water molecules are even smaller than the holes, so they can pass through. Sugar molecules are much bigger than the holes, so they cannot pass through. d When it is placed in pure water, an animal cell absorbs water by osmosis. This is because there is a higher water potential outside the cell than inside it. The extra water makes the cell swell, until it bursts. e Plant cells are held in shape by their full vacuoles, which push outwards against the strong cell wall, producing a very firm structure. A plant cell like this is said to be turgid. Turgid cells pressing against each other make plant tissues strong and firm. When the cells are not full of water, they are no longer turgid, and their contents do not press outwards on the cell wall. The cells, and the tissues in the leaves that they make up, become soft and floppy. This is why the plant wilts. 4 athe movement of molecules / ions, down a concentration gradient / from a high concentration to a low concentration; as a result of their random movement; [2] b i 70 Time for litmus to go blue / s randomly back and forth through the holes. Because there are more of them in the water than in the sugar solution, their net movement will be into the tubing. So the corrected sentence could be: Answers to end-of-chapter questions 60 50 40 sample A 30 20 sample B 10 0 0 2 4 6 8 10 12 Distance along tube / cm 14 16 all points correctly plotted; lose one mark for any incorrect point neat best-fit line drawn; [3] ii ammonium hydroxide is alkaline; [1] iii A; [1] iv C’s concentration was between A and B; specific evidence quoted to support this statement, e.g. it took less time for it to travel 10 cm than A and more time than B.[2] 5 a the pH is greater than 8; [1] b table is drawn with a ruler and has rows and columns for dimensions of block and time taken to go colourless; headings for both quantities include correct units – including time / s; times to go colourless are correctly recorded as 128 and 72 (with no units); [3] 2 © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences c i diffusion; [1] iihydrochloric acid neutralised the alkaline substance in the agar / the pH became less than 8; [1] d block B had a greater surface area to volume ratio / the distance for diffusion to the centre of the block was smaller in block B;[1] Chapter B3 Biological molecules 1 amonosaccharide, found in both plants and animals, used as fuel in respiration b polysaccharide, found in plants only, used as an energy store in plant cells c polysaccharide, found in plants only, used to make cell walls d polysaccharide, found in animals only, used as an energy stores in (liver) cells 2 a b c d e f nitrogen (or sulfur) amino acids Benedict’s lipid (fat) sucrose sucrose metabolism or metabolic reactions 3 Measure equal volumes of each solution into two identical test tubes. Add equal volumes of Benedict’s solution to each one. Place both tubes into a water bath at about 80 °C. Do this at exactly the same time. Watch carefully. The one that changes to green or orange first, or the one that is the darkest orange after a set length of time, is the one that has the most concentrated solution of reducing sugar. 4 Substance Elements Carbohydrate, How to One it contains fat or protein? test for it function haemoglobin C, H, O, N 3 protein biuret test carries oxygen in the blood glucose C, H, O carbohydrate Benedict’s to provide test energy starch C, H, O carbohydrate iodine test stores energy in plant cells enzyme C, H, O, N protein biuret test speeds up reactions Answers to end-of-chapter questions 5 a a protein catalyst, which speeds up the rate at which metabolic reactions take place b a term used to describe the state of a protein molecule that has lost its shape – often caused by high temperature or extremes of pH; a denatured enzyme molecule is unable to catalyse its reaction because the substrate no longer fits into its active site c the substance that is changed into products by an enzyme; the substrate fits into the enzyme’s active site d a new substance formed in an enzyme-catalysed reaction e the part of an enzyme molecule into which a substrate molecule fits 6 a About 37 °C – human body temperature. b About 2 – hydrochloric acid has a very low pH. c At low temperatures, molecules have low kinetic energy and move slowly. This means that the frequency of collisions between enzyme molecules and substrate molecules is also low. d Above the enzyme’s optimum temperature, the enzyme molecule begins to lose its shape – it is denatured. This means that the substrate molecule does not fit into the active site, so the enzyme cannot catalyse the change of the substrate into products. 7 a b c d calcium; water; they both contain protein; orange-brown; it does not contain starch; [1] [1] [1] [2] e protein, fat and carbohydrate; [1] 8 a blue-black; bthe blue-black colour would have disappeared from parts of the plain paper. c i Time / Number of new Total number of minutes areas where there areas where there had been a reaction had been a reaction 1 14 14 2 28 42 3 18 60 4 12 72 5 6 78 [1] [1] [2] © Cambridge University Press 2017 ii Number of new areas where there had been a reaction Cambridge IGCSE Combined and Co-ordinated Sciences 30 20 10 0 0 1 2 3 Time / minutes 4 5 time on x-axis and number of new areas on y-axis; scales on both axes go up in even steps (e.g. 1, 2, 3 etc. on x-axis, 10, 20, 30 etc. on y-axis); both axes fully labelled including units; all points accurately plotted with small, neat crosses or circles with a ring around them; straight lines drawn between the points / good best-fit line drawn; [5] iii any two sensible suggestions about differences between the goats, e.g. different ages, different genders, different breeds, different concentrations of enzyme in their saliva, how hungry they were when the saliva was collected; [2] d continue for longer; take readings more often than one minute intervals; include some discs that have no enzyme in them / have boiled enzyme in them; repeat the experiment two more times; [max 3] 9 a sucrose molecules and enzyme molecules move randomly; sucrose molecule collides with enzyme’s active site; enzyme causes sucrose molecule to split into glucose and fructose; reference to involvement of water in this reaction; products / glucose and fructose, leave the active site; [max 3] b i optimum temperature for enzymes; temperature kept constant because, pH is the independent variable / temperature is a control variable; [2] ii no activity below pH 3; optimum / greatest activity, is at pH 7; no activity above pH 11; [3] Chapter B4 Plant nutrition 1 4 Obtained from Used for Nitrates the soil making amino acids and proteins Water the soil photosynthesis, maintaining turgor / supporting tissues, transporting substances Magnesium the soil making chlorophyll Carbon dioxide the air photosynthesis Answers to end-of-chapter questions 2 a A chloroplast is an organelle that contains the pigment chlorophyll. Photosynthesis takes place inside chloroplasts, with the help of chlorophyll, which absorbs energy from light. b The palisade mesophyll is closer to the upper surface of the leaf than the spongy mesophyll. The cells in the palisade mesophyll are tall and thin, while the cells in the spongy mesophyll are more rounded. The palisade cells contain more chloroplasts than the spongy cells. More photosynthesis takes place in palisade cells than in spongy cells. There are larger air spaces in the spongy mesophyll than in the palisade mesophyll. c Organic substances have been made by living organisms, e.g. carbohydrates, proteins, vitamins. Inorganic substances have not been made by organisms, e.g. magnesium ions, water. d Guard cells are pairs of sausage-shaped cells found in the epidermis of leaves (usually in the lower epidermis). The hole in between the pair of guard cells is a stoma. 3 a carbon dioxide + water → glucose + oxygen b Carbon dioxide enters the leaf through stomata, by diffusion from the air. Water enters the root hairs, by osmosis from the soil and is then transported up the xylem to the leaf. c Glucose is used to make starch, or to provide energy by respiration. Oxygen diffuses out of the leaf into the air, through the stomata. 4 a Carbon dioxide diffuses through the stoma and then through the air spaces, allowing it to reach the cells in the palisade layer. Oxygen diffuses in the opposite direction when photosynthesis is taking place. (When you have learnt about transport in plants, you will also find out that the air spaces are important for allowing the movement of water vapour out of the leaf.) b This means that light can pass straight through these cells, so little light is lost before it reaches the palisade cells, where it is used in photosynthesis. c The larger the surface area, the more sunlight will hit the leaf. This means that more energy can be absorbed by chlorophyll, so more photosynthesis can take place. d The veins bring water from the soil to the leaf cells. By branching, they can bring water close to every cell. The cells need water for photosynthesis, and to maintain their turgor, helping the leaf to be held out straight. 5 a sucrose. This is a soluble sugar, which can dissolve in water for transport. It is not too reactive. © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences b starch. This is an insoluble polysaccharide, which can be stored as solid grains in cells and will not interfere with the reactions that take place in the cell. (It also does not affect the water potential of the cell; if sucrose was stored, this would tend to draw excess water into the cell by osmosis.) 6 a Leaf from plant A: all orange-brown; Leaf from plant B: uncovered part blue-black; covered part orange-brown; b i to break down the cell membranes so that iodine solution and starch can come into contact; [1] ii to remove the chlorophyll; c i [3] [1] cover other areas with a simlar material that is transparent (so that the only difference is whether light can reach the leaf); [1] ii it controls a significant variable – having different plants could affect the results / because one plant might respond differently from another; [1] d use a plant with variegated leaves; destarch it; then leave in the light long enough for it to make starch; test a leaf for starch; would expect green parts to go blue-black, white parts to be orange-brown; [max 3] 7 a i F; [1] ii A; [1] iii D. [1] b i little light is lost before it reaches the palisade cells, where it is used in photosynthesis; [1] ii the waxy cuticle prevents water loss through this surface of the leaf; [1] iii bring water to the leaf; take sucrose away from the leaf; help to support the leaf. [max 2] c i carbon dioxide; water [2] ii some is used in respiration to release energy; some is converted to starch for storage; some is used to make cellulose cell walls for new cells; some is converted to sucrose for transport to other parts of the plant; some is converted, with the addition of nitrogen, to amino acids; some is converted to, fats / lipids [max 4] Chapter B5 Animal nutrition 1 a i calcium, vitamin D ii carbohydrate, fat, protein 5 Answers to end-of-chapter questions iii protein iv fibre v protein, iron vi vitamin D b There is a very wide range of possible answers. Images B5.02 to B5.04, and Tables B5.02 and B5.03, provide some examples. Answers can also be checked against a table of nutrient values of foods. Search on the internet for: 'food nutrient content table' and select one that covers foods commonly eaten in the relevant country. 2 a Digestion is the breaking down of large food molecules into small ones. Absorption is the movement of these small molecules through the wall of the small intestine and into the blood. b The small intestine is longer and narrower than the large intestine. It is made up of the duodenum and ileum, whereas the large intestine is made up of the colon and rectum. Digestion and absorption of all types of food molecules – including water – takes place in the small intestine. Only water absorption takes place in the large intestine. c Enamel is the exceptionally hard outer layer of a tooth. Dentine is a softer layer beneath the enamel. Dentine contains living cells, but enamel does not. d Bile is a greenish liquid made in the liver and stored in the gall bladder, whereas pancreatic juice is made in the pancreas. Both liquids flow along ducts into the duodenum. Bile contains bile salts, which are not enzymes but which help to emulsify fats (break large droplets into small ones). Pancreatic juice contains several different digestive enzymes that digest fats, proteins and carbohydrates. Both bile and pancreatic juice also contain sodium hydrogencarbonate, which neutralises the acid from the stomach. 3 a A salivary gland B oesophagus C stomach D pancreas E duodenum F ileum G colon H rectum I anus J liver b i A and D ii C and D © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences Answers to end-of-chapter questions iii D diagram shows a molar tooth; correct labels to: enamel; dentine; pulp cavity; nerves and blood vessels; crown / root; iv C v F and G vi I 4 ingestion amylase starch mucus oesophagus hydrochloric proteins duodenum small pancreas gall fatty acids glycerol 5 a vitamin C and vitamin D; [1] b they all already are small molecules; which can pass through the walls of the ileum; [2] c any two dairy foods, bread; [1] d helps calcium to be absorbed; needed for making, bones / teeth; [2] e anaemia; lack of energy; iron is needed to make haemoglobin; which transports oxygen around the body; lack of oxygen means less respiration; [max 3] 6 a A incisor; B canine; C molar; [3] b tooth A: cut off pieces of food; to help with ingestion; tooth C: crush / grind, food; to increase surface area for enzyme action; [4] c 7 a i blue-black; [6] [1] ii starch is present; [1] iii rows 2, 3 and 4 show sugar absent, starch absent and sugar absent; b i rows 5, 6, 7 and 8 show starch absent, sugar present, starch absent, sugar present; [2] breaks down starch to sugar; [1] ii results show there is sugar in the water in the beaker; so sugar molecules have moved through the membrane; sugar molecules are small enough to pass through the holes in the membrane; [2] small intestine / duodenum / ileum; [1] c i ii blood / blood plasma / capillaries; [1] d its molecules are too big to be absorbed / to pass through the wall of the small intestine; [1] 8 a breakdown of large / insoluble molecules; to small / soluble molecules; [2] b amylase; [1] c i [1] at the beginning; ii maltose; [1] iii line is of similar shape; line is above the 35 ºC line; [2] d to produce molecules that are small enough to be absorbed / because starch molecules are too large to be absorbed; [1] Chapter B6 Transport in plants 1 a b c d e f 6 xylem vessel xylem vessel root hair transpiration stoma potometer © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences b so plants can make more amino acids / proteins; 2 a and b root hairs liquid root cortex cells liquid xylemliquid leaf mesophyll cells liquid air spaces in leaf gas stomata gas to make more cells for growth; 1 a vena cava, right atrium, right ventricle, pulmonary artery, lungs, pulmonary vein, left atrium, left ventricle, aorta b vena cava, right atrium, right ventricle, pulmonary artery b, c and d 4 a 20; [1] b as wind speed increases, water uptake increases; any use of manipulated figures (e.g. doubling of wind speed from 2 to 4 metres per second results in 1.7 times the rate of water uptake); [2] c light intensity; temperature; humidity; [max 2] more root hairs; shorter root hairs; decrease in length of root hairs is (much) greater for plant B;[2] iii less surface area; so less uptake of mineral ions / water; so less photosynthesis; less glucose / starch / carbohydrate synthesised; so less fuel for respiration / less energy available; less nitrate reduces protein synthesis; 7 2 a Arteries take blood away from the heart; veins take blood towards the heart. Arteries have thick, elastic walls; veins have thinner walls. Arteries have a narrow lumen; veins have a wider lumen. Arteries do not have valves; veins have valves. b Oxygenated blood contains a lot of oxygen (combined with haemoglobin inside the red blood cells) and is bright red. Deoxygenated blood contains less oxygen, and is a duller purplish-red. c An atrium is one of the upper chambers of the heart, which receives blood and which has thin walls. A ventricle is one of the lower chambers of the heart, which has thick walls that pump blood out of the heart. d A red blood cell is a small cell with no nucleus, indented, and containing a large amount of haemoglobin. Its function is to transport oxygen. There are several types of white blood cells, but most are larger than red blood cells and they all have a nucleus. They do not contain haemoglobin. Their function is to fight pathogens. 3 a b c d e 4 [2] ii both show same increase in number of root hairs (per unit area); [2] Chapter B7 Transport in animals 3 a a section cut across something 5 a i Answers to end-of-chapter questions [max 3] plasma white cells red cells platelets and plasma plasma • Arteries: thick walls to withstand high-pressure blood; elastic walls to withstand pulsing blood; narrow lumen so blood moves through fast • Veins: valves to keep low-pressure blood moving in one direction; wide lumen to provide least resistance to blood flow • Capillaries: very narrow, so red blood cells have to squeeze through and are brought close to cells that require oxygen; very thin walls with gaps, so substances can easily move between blood and tissue fluid • Xylem vessels: dead and hollow so nothing in the way of water movement; narrow, so a tall column of water © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences can be supported without breaking; lignin in walls to make them waterproof and to provide strength; pits in walls to allow water to move sideways • Phloem tubes: living but with no nucleus and only a small amount of cytoplasm, so sap can flow through; perforated end walls to allow sap to flow through 5 a contains haemoglobin that combines with oxygen; collects oxygen in lungs, releases it in body tissues; [2] b protects against, disease / pathogens; takes in and kills micro-organisms / bacteria / pathogens;[2] c to deliver requirements to body cells; e.g. oxygen / glucose / other named nutrient; to remove waste products from body cells; e.g. carbon dioxide / other named waste product; [max 2] 6 a Red cell in diagram measures 23 mm; so magnification = 23 / 0.007; = × 3285. b it has no nucleus; [3] it has a depression in the centre / is a biconcave disc; it contains haemoglobin. c i [3] transporting oxygen; ii it contains haemoglobin; which combines reversibly with oxygen; it has a large surface area to volume ratio; which speeds up the movement of oxygen into and out of the cell; it is small; which allows it to squeeze through very small capillaries; it has no nucleus; which makes more room for haemoglobin. [max 3] 7 a 2; [1] b i [1] about 0.75 s; ii explanation of measuring time between two equivalent points; [2] c ventricle volume decreasing; because the muscle is contracting;[2] d when the ventricle contracts, the valve shuts; because of the pressure of the blood pushing upwards on it; when ventricle relaxes, valve opens; [3] e line follows the same pattern as the first, at the same times, but does not rise to such a high volume; [2] 8 a A left atrium; B bicuspid valve / atrioventricular valve; C semilunar valve; D right ventricle; 8 [4] Answers to end-of-chapter questions b E vena cava; F aorta; [2] c coronary (arteries); plaques / cholesterol / fat deposit, in artery wall; partly blocks artery; less blood can flow through; less oxygen carried to heart muscle; increased likelihood of blood clotting; [max 3] d to keep the blood moving; to keep the blood oxygenated; to remove carbon dioxide from the blood; [max 2] e has a septum dividing the two sides of the heart; oxygenated blood on the left and deoxygenated on the right; both sides contract at the same time; more muscle on the left side; so more pressure produced on the left side; high pressure to most of body; low pressure to lungs; [max 4] Chapter B8 Gas exchange and respiration 1 a protein synthesis, cell division, growth, movement, passage of nerve impulses, maintaining a constant body temperature b respiration c glucose + oxygen → carbon dioxide + water 2 a inspired air has more oxygen; inspired air has less carbon dioxide; inspired air usually has less water vapour b Oxygen is used by body cells in respiration. Carbon dioxide is produced by body cells in respiration. Water evaporates from the lining of the lungs into the air, so it is breathed out in expired air. 3 a the movement of oxygen into the body and the loss of carbon dioxide b the alveoli in the lungs b any three of: large surface area; thin; good supply of air containing oxygen; good blood supply 4 a b c d anaerobic both aerobic only in humans; both in yeast both 5 The following sequence should be shown, in a diagram or words: down trachea, bronchus, bronchiole, into alveolus (by mass flow of air), across wall of alveolus into the blood, by diffusion into a blood capillary into a red blood cell, combines with haemoglobin, carried along the pulmonary vein to the left atrium of the heart then to the left ventricle, pumped out of the heart into the aorta, then to a capillary in the arm muscle, diffuses out of the red blood cell, diffuses out of the capillary, diffuses into the muscle cell © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 6 a i to make sure all the carbon dioxide had been removed;[1] ii clear; b i [1] to see if any carbon dioxide had been produced;[1] Answers to end-of-chapter questions most of the graph paper provided; each bar drawn neatly and precisely; [4] b the more cigarettes smoked per day, the greater the chance of dying between the ages of 40 and 60 years old; the younger a person is when they start smoking, the greater the chance of dying between the ages of 40 and 60 years old; ii cloudy; [1] c have another apparatus in which flask 4 has no insects;[1] d i red / orange; [1] ii carbon dioxide present; 7 a b c d e f Chapter B9 Coordination and homeostasis dissolves / reacts with water; to produce an acidic solution; e respiration ; [3] [1] 12; [1] 21; [1] 3 0.5 dm ;[1] 1.1 dm3;[1] more rapid breathing brings fresh air into the lungs more often; deeper breathing brings a larger volume of fresh air into the lungs; more oxygen can diffuse into the blood more quickly; supplying more oxygen to the muscles; so they can respire faster; releasing more energy from glucose; [max 4] brain senses the pH of blood; pH decreases during exercise; because more carbon dioxide is dissolved in the blood plasma; brain responds by sending more frequent impulses to the breathing muscles; so they contract harder and more frequently; [max 4] 8 a 12.5 breaths per minute at start, 25 breaths per minute during exercise; so increase is 12.5 breaths per minute; [2] b from just before 11 minutes to just before 16 minutes; 5 minutes; [2] c during exercise not enough oxygen was supplied to muscles; so they respired anaerobically (as well as aerobically); producing lactic acid; which was broken down by combining with oxygen (when exercise finished); reference to paying back the oxygen debt ; [max 4] d would follow a pattern similar to that of breathing rate; heart pumps oxygenated blood to the muscles; more oxygen required by muscles as they exercise; so that they can respire faster; more carbon dioxide needs to be removed from the muscles; continuing need for more oxygen after exercise to pay off oxygen debt; [max 4] 9 a axes correctly labelled; x-axis scale uses the ranges from the table; good scale on both axes that uses 9 the number of cigarettes smoked per day seems to increase the chance of dying between 40 and 60 more than the age at which smoking started; [3] 1 a a reflex action b The stimulus from the sharp object is detected by a receptor in the foot. This sends an electrical impulse along a sensory neurone to the brain or spinal cord. The impulse is passed along a relay neurone and then to a motor neurone. This transmits the impulse to an effector, the muscles in your leg, and makes them contract. 2 a b c d e motor and relay sensory sensory motor, relay relay 3 a b c d e f motor neurone receptor cornea retina contraction circular 4 a Keeping the body temperature constant is just one part of homeostasis, which is the maintenance of a constant internal environment. Homeostasis also involves the regulation of blood glucose concentration, as well as the water content of the body. b The hairs do stand on end when the body is too cold, but in humans we do not have enough hair for this to help to keep us warm. In other hairier mammals, the raised hairs trap a layer of insulating air next to the skin. c Air of any kind cannot get into the body through the skin. The fat layer prevents heat leaving the body by conduction, as it is an insulator. d The sweat glands do secrete sweat onto the surface of the skin when we are too hot, but this liquid is not cold. It cools the body because the water in the sweat evaporates, and this process takes heat energy from the skin. © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences e The blood capillaries do not move at all. The arterioles that supply the blood capillaries near the surface of the skin get wider (dilate) when you are too hot. This allows more blood to flow through these capillaries, allowing heat to radiate from the blood into the air. f Insulin is a hormone, not an enzyme. Enzymes catalyse reactions, but insulin is not a catalyst. Insulin causes enzymes in liver cells to convert glucose to glycogen. 5 a A 37.4 °C; B 37.5 °C. [2] b homeostasis; humans are endothermic; body produces more heat to maintain body temperature; shivering; vasoconstriction; [max 4] c air is more insulating than water; heat lost more easily from the body in water than in air; by conduction; [max 2] d person A was moving but person B remained still; idea that ‘new’ cold water was constantly coming into contact with A’s skin; water around B’s body warms up (as heat is lost from his body to the water); heat transfers from hot object to cold object; so more heat lost from A’s body than B’s body; [max 3] 6 a for respiration; by combining it with oxygen to provide energy; (not ‘produce’ energy) for named function (e.g. movement, active transport); [max 3] b pancreas; [1] c i starch digested to glucose; by enzymes / amylase and maltase; absorbed into the blood from, the small intestine / ileum; [3] ii insulin secreted; causes liver to take up glucose from the blood; liver converts glucose to glycogen; (also) glucose used by body cells in respiration; [max 3] d negative feedback is a process that brings concentration back to normal when it gets too high or too low; when blood glucose concentration rises too high, insulin is secreted and reduces it to normal; when blood glucose concentration drops too low, glucagon is secreted and raises it to normal; [3] 7 a ability to detect changes in the environment; and respond to them; b i gravitropism; firm anchorage in the soil; leaves have more light; 10 for photosynthesis; [2] iii builds up on the lower side; causes cells in stem to elongate more; causes cells in root to elongate less; 1 a b c d e f g 2 gamete zygote asexual pollination seed fertilisation sexual Asexual reproduction Sexual reproduction only one parent involved all offspring genetically identical one or two parents involved involves gametes involves fertilisation zygote formed genetic variation among offspring 3 a i age of seeds; [1] ii water; oxygen; warm temperature; [3] (if light also given, max 2 marks) b i young plants will get light for photosynthesis; [1] ii D;[1] 4 a i a sex cell; joining together of nuclei of male and female gametes;[2] ii A – sepal; B – produces pollen; iii wall of ovary; b B and C; [2] [1] they have water; they have a suitable temperature; they do not need light; [max 3] tropism; [2] [1] ii better photosynthesis; [2] [3] Chapter B10 Reproduction in plants c i ii better access to water; Answers to end-of-chapter questions negative gravitropism; because leaves can get more light; flowers held up higher; where insects can access them; [2] [max 3] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 5 a asexual; [1] b produces new banana plants that are identical to the parent (so the bananas will be exactly the same variety); produces large new plants quickly; [2] c all new plants will be genetically identical; if the parent did not have resistance to the disease then nor will the offspring; [2] Answers to end-of-chapter questions 4 a fetus to mother: carbon dioxide and urea; nucleus cytoplasm cell membrane [2] flagellum digestive enzymes Chapter B11 Reproduction in humans 1 a b c d oviduct ovary uterus cervix all five labels correct three marks 2 a A uterus wall B oviduct C amnion D amniotic fluid E fetus F placenta G umbilical cord H cervix I vagina b It produces amniotic fluid, in which the fetus floats. This fluid protects it from bumps and knocks. c The placenta brings the mother’s and fetus’s blood close together, but does not allow them to mix. In the placenta, useful substances such as oxygen and glucose diffuse from the mother’s blood to the fetus’s blood. Wastes such as urea and carbon dioxide diffuse from the fetus’s blood to the mother’s blood. 3 a i they are haploid / they have only one set of chromosomes;[1] ii it contains food stores for the developing embryo; [1] iii the food stores will soon run out; (once attached) it obtains nutrients; and oxygen; from the mother’s blood; through the placenta; [max 4] b i A umbilical cord; B amnion; C cervix; [3] ii the (beating of the) fetus’s heart; [1] iii support / protect, the fetus; [1] iv mother to fetus: any two of oxygen / glucose / amino acids / water / other named soluble nutrient;[2] 11 four labels correct two marks two or three labels correct one mark [3] b acrosome contains enzymes which digest through the jelly surrounding the egg; mitochondria release energy by aerobic respiration (for swimming); flagellum propels the sperm forwards; nucleus contains the haploid number of chromosomes so the normal diploid number is restored at fertilisation; shape is streamlined to reduce energy needed for swimming; [max 4] c nucleus contains the haploid number of chromosomes so the normal diploid number is restored at fertilisation; it contains food stores to provide for the young fetus (until it is implanted); it is surrounded by a protective layer of jelly; [3] 5 a increased and then decreased; peaks in 2004 and 2007; any figure quote using both year and number of people infected read from the graph; [3] b people recently infected with HIV show no symptoms; may not have had their blood checked; [2] c more awareness of AIDS; people with HIV/AIDS now knew that they had it and avoided passing it on; people who were not HIV positive modified their behaviour to reduce the risk of becoming infected with HIV; example – avoided having multiple partners / used condoms / did not share contaminated needles; use of antiretroviral drugs to treat AIDS; other valid point. [max 4] Chapter B12 Inheritance 1 a a large letter for the smooth fur allele and a matching small letter for the rough fur allele, using letters that look different from each other, e.g. A and a (not S and s) b AA, Aa and aa c AA smooth fur, Aa smooth fur, aa rough fur © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 2 a a large letter for the red colour allele and a matching small letter for the white colour allele, using letters that look different from each other, e.g. R for the red colour allele and r for the white colour allele b R is dominant, because this is the allele that has an effect in a heterozygous plant. c RR, red; Rr, red; rr, white 3 a A gene is a length of DNA that codes for a particular protein; an allele is one of two or more forms of a gene. b A dominant allele shows its effect in a heterozygous organism; a recessive allele only has an effect when no dominant allele is present. c A homozygous organism has two identical alleles of a gene, e.g. AA; a heterozygous organism has two different alleles of a gene, e.g. Aa. d The genotype shows the alleles of a gene that an organism possesses; the phenotype describes the characteristics of the organism. e Mitosis is a type of nuclear division in which genetically identical daughter cells are produced; meiosis is a type of nuclear division that produces daughter cells with only half the full number of chromosomes, and that are genetically different from one another. Mitosis is used in growth, repair and asexual reproduction; meiosis is used to produce gametes. f A haploid cell has one full set of chromosomes; a diploid cell has two full sets. 4 a symbols should be the same letter, large and small, and easily distinguishable, e.g. EE for indented edges; ee for smooth edges; c b parents’ phenotypes indented smooth parents’ genotypes EE ee gametes E e all Ee indented parents’ genotypes correct; gametes correct and placed inside circles; offspring genotype and phenotype correct; entire genetic diagram laid out correctly with all headings;[4] parents’ phenotypes indented indented parents’ genotypes Ee Ee E and e E and e gametes spring genotypes and phenotypes E e E EE indented Ee indented e Ee indented ee smooth parents’ genotypes correct; all gametes correct and shown inside circles; genotypes of offspring correct; phenotypes of offspring correctly associated with genotypes; 99 : 302 is approx. 3 : 1 and genetic diagram shows 3 indented : 1 smooth; [5] 5 a i white is dominant and himalayan is recessive – no mark upper case and lower case version of the same letter chosen; upper case for white and lower case for himalayan;[2] ii parents’s genotypes shown as Aa and Aa (or whatever letters have been chosen in part a); gametes from both parents shown as A and a with circles around them; offspring genotypes shown as AA, Aa, Aa and aa; AA and Aa offspring identified as white, and aa as himalayan; ratio stated as 3 : 1 white to himalayan and matched to three quarters white and one quarter himalayan;[5] [2] spring genotypes and phenotypes 12 Answers to end-of-chapter questions b i respiration; oxidation of glucose / equation; [2] ii air trapped between hairs; insulation; reduces heat loss; [max 2] iii extremities / ears/ paws / nose, colder than other parts of the body; enzyme active only in these parts so black pigment only produced there; [2] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences Chapter B13 Variation and natural selection Answers to end-of-chapter questions b i 1 species, discontinuous, genes, continuous, mutation, adapted 2 a In continuous variation, an individual can fit anywhere within a range of a particular characteristic, with no sharp dividing lines. In discontinuous variation, there are a small number of distinct categories into which any individual fits. b Natural selection is the increased chances of individual organisms with particular variations surviving and reproducing in their environment, because of selection pressures that act on them. Artificial selection is the choice, by humans, of individuals with particular variations to be allowed to breed together. 3 a Sexual reproduction allows mixing of alleles from different parents. There is genetic variation in the population. Different combinations of alleles may give different features that make some individuals better able to survive and reproduce in the changing environment than their parents. Asexual reproduction, however, produces offspring with exactly the same combinations of alleles as their parent; there is no genetic variation. 4 a correct answer given (you will need to get someone to check!); [1] shape of ear lobes shows discontinuous variation; so it is caused by genes [2] ii approximately 3 : 1; free : attached; [2] iii allele for free ear lobes likely to be dominant; and allele for attached ear lobes likely to be recessive;[2] 5 a i There are no distinct categories; individuals can have any wing length within the range from 63 mm or less to 70 mm or more; [2] ii for example: body mass / body length / beak length;[1] 13 ii repeat measurements for a larger number of birds; repeat in countries other than Sweden; check wing lengths of birds that are breeding; follow individual marked birds throughout their lives to measure wing length and length of life; measure the wing length of dead birds; [max 3] c birds with this wing length survive for longer; more likely to reproduce than birds with smaller wings; wing length determined by genes / alleles which are passed on to offspring; [max 4] Chapter B14 Organisms and their environment (In both sexual and asexual reproduction, mutation may occur, which could form new alleles that might give an advantage to an organism and be selected for. This is no more likely in sexual than in asexual reproduction.) b Mutation may produce new alleles that were not present before. Although mutations usually produce new characteristics that are less good than the normal ones, just occasionally a new feature that gives an organism a survival advantage may occur. If so, then this will be selected for (its owners will be more likely to survive and reproduce) and passed on to the next generation. b i the largest number of birds trapped has wing lengths of 66 or 67 mm; suggesting that most birds had these wing lengths; comparative data quoted for birds with these wing lengths and others; birds with these wing lengths had greater mean ages when trapped; suggesting that they lived longer than others; comparative age data quoted for birds with these wing lengths and others; [max 4] 1 a A producer is an organism that makes its own organic food materials from inorganic ones; plants are producers, as they make organic nutrients by photosynthesis. A consumer is an organism that depends on organic nutrients made by producers; animals and fungi are consumers. b A primary consumer obtains its energy by feeding on plants; it is a herbivore. A secondary consumer obtains its energy by feeding on primary consumers; it is a carnivore. c A food chain shows how energy is transferred from one organism to another, showing only one species at each trophic level. A food web shows many interlinking food chains, with more than one species shown at each trophic level. 2 a to make carbohydrates, fats and proteins b by photosynthesis; carbon dioxide from the air is used to make carbohydrates c They are given out from the plant as carbon dioxide. d They break down carbohydrates, fats, proteins and other carbon-containing materials in dead organisms or waste products from them; they then respire, giving out carbon dioxide to the atmosphere. 3 a i sunlight; ii chemical energy; b i respiration; [1] [1] [1] ii movement / muscle contraction; active transport; generating heat to keep the body warm; transmission of nerve impulses; building large molecules from small ones; [max 3] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences c i the food web should show an arrow going from the wildebeest to ticks, another arrow going from the ticks to the oxpeckers; and an arrow going from the wildebeest to the oxpeckers; [1] ii energy is lost between trophic levels; 90% of energy lost / only 10% of energy passed on; lost, in respiration / as heat; so fewer organisms can be supported at each trophic level; [max 3] 4 a photosynthesis by aquatic plants; dissolving from the air; [2] b bacteria feed on the sewage; so their populations increase; bacteria respire; aerobically; use up oxygen from the water; [max 4] 14 Answers to end-of-chapter questions c i increasing quantities of untreated sewage running into the river; build-up of nutrients in the water; so larger bacteria populations used up more oxygen; [max 2] ii sewage treated before entering the river; fewer nutrients for bacteria; so fewer bacteria / less use of oxygen by bacteria; [max 2] d they would die / leave the river; [1] e cause unpleasant smells; introduce pathogens to the water that could cause disease in humans; e.g. cholera bacterium; e.g. polio virus; other example of water-borne disease-causing organism; [max 2] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences Chemistry Answers to end-of-chapter questions Chapter C2 The nature of matter 1 a Both ways of categorising substances have their use to a chemist. Chapter C1 Planet Earth 1 a i • Knowing whether a substance is a solid, liquid or gas at room temperature – and how easily a substance can change its state – helps us in handling the different substances and in separating them and purifying them from mixtures. It is important to realise that any substance can exist in any of the three states, depending on the conditions of temperature and pressure. nitrogen 78% Percentage in unpolluted air oxygen 21% other gases 1% [2] ii carbon dioxide, argon, helium b i N2 + O2 → 2NO [1] [2] ii that natural rain water is slightly acidic [1] / from dissolved carbon dioxide [1] / after thunderstorm more acidic because of dissolved nitric acid [1] [3] 2 a use cobalt chloride paper – turns from blue to pink; or use anhydrous copper sulfate powder – turns from white to blue [2] b as a coolant, or any other correct industrial use [1] c a substance that dissolves another to form a solution [1] d i burning coal in power stations or other correct source [1] ii kills fish in lakes, erodes statues/buildings [2] iii 64 e 1: filtration to remove solid particles 2: chlorination to kill bacteria/germs f 21% [1] [2] [2] [1] 3 a lower proportions of oxygen and nitrogen; higher proportion of carbon dioxide [1] b acid rainfall causing damage to trees [1] / acidification of lakes [1] [2] c add drops of the liquid to anhydrous copper(II) sulfate powder (1); powder turns from white to blue (1) OR add drops of the liquid to cobalt chloride paper (1); colour change from blue to pink (1) [2] d methane [1] e burning of fossil fuels / volcanic activity [1] f neutralisation [1] g heating (thermal decomposition of) limestone in furnace (kiln) [1] CaCO3 → CaO + CO2[2] 15 • Knowing whether a sample is an element, compound or mixture helps us in knowing and predicting the chemical properties of a substance. These distinctions are mutually exclusive and therefore are more fundamental to our understanding. b The word ‘particle’ is needed when talking in generalisations about the structure and movement of the constituents of matter. The context should always be defined to distinguish this scientific use of the word from the more everyday use when it can be a speck of dust, etc. The one key experiment where the two uses interact is in the description of Brownian motion. Here the unseen motion of atoms and molecules in a fluid is demonstrated by the jerky, random motion of the dust particles as they are hit by the submicroscopic particles that make up matter. One aspect that can be discussed, and needs to be referred to, is the key definition of the size of the ‘particles’ involved when the term is used. Descriptions such as ‘sub-microscopic’ and ‘subatomic’ are useful. 2 a i The particles are in fixed positions [1]; they vibrate about their fixed position [1]. [2] ii Add water, stir to dissolve salt and filter to obtain sand as the residue. [3] b distillation, lower, volatile, condenser, vapour [5] 3 a seawater b evaporation [1]; freezing (solidification) [1] [1] [2] 4 a A: thermometer; B: beaker b to keep the temperature the same throughout [2] [1] c i 48 °C [1] ii 72 °C d The particles are close together but irregular [1]; the molecules are able to move about with slow movement [1]. [1] [2] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences e i The third statement is correct: its melting point is different from pure stearic acid. [1] ii in testing medicines or food additives, or other correct[1] 5 a balloons b i nucleus [1] [1] ii The third statement is correct: helium has a complete outer shell of electrons. [1] iii 34 [1] 34 iv 18 Ar [1] c The atoms are arranged irregularly [1] and are close together/touching [1]. [2] 6 a electrons b P has 2 protons and 2 neutrons (= 4 nucleons) Answers to end-of-chapter questions 4 a i ii E iii F iv B v A[5] b i Correct electron structure of the F ions (electrons from outer orbit of C moved to the two F atoms, one electron to each to give eight electrons in the outer shell of each) [1] Correct charges on each ion: – on F and 2+ on C[2] − [1] F [1] c atoms are electrically neutral because they have equal numbers [1] of protons and electrons [1] D [C]2+ − F [2] d R[1] e 2.2 [1] ii high melting point, soluble in water, conducts when dissolved or molten, brittle (any two of these possible answers) [2] [3] 5 a i Chapter C3 Elements and compounds 1 a helium / aluminium / chlorine b i B and C[1] ii C[1] iii D[1] 2 a Period 2 b i [1] O ii F iii Li v Be vi N c atoms, protons [6] [2] 3 a hydrogen [1] P is in Group I / Q is in Group VIII (or 0) / R is in Group VII [1] the Group number is given by the number of outer electrons in the atom [1] ii Q is the least reactive as it is a noble gas [2] [1] iii P is a good conductor of electricity as it is a metal[1] 16 ii Y reacts with X, Z does not b i the elements are too reactive [1] [1] ii An electron is transferred (donated) from a sodium atom to a chlorine atom [1]; the sodium becomes a positive ion and the chlorine a negative (chloride) ion [1] [2] Chapter C4 Chemical reactions iv C b i X conducts electricity, Z does not; or X reacts with water, Z does not [1] 1 a There is a colour change which shows that there might be a reaction, and new substance(s) are formed / a gas is given off. b The most reliable evidence for a chemical reaction is that a gas is given off which can be identified as carbon dioxide. c copper carbonate → copper oxide + carbon dioxide zinc carbonate → zinc oxide + carbon dioxide d Zinc oxide is a white solid which turns yellow when heated. When cooled, the solid turns white again. e No, it is a physical change. © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 2 a black solid b magnesium + carbon dioxide → magnesium oxide + carbon c i carbon dioxide ii magnesium d MgO + 2HCl → MgCl2 + H2O [correct formulae but unbalanced = 1] e i Zn2+ + Mg → Mg2+ + Zn [1] iii lime/calcium oxide, or slaked lime/ calcium hydroxide d Fe + 2HCl → FeCl2 + H2 [1] 3 Step 2: Filter to remove excess solid [1] [1] Step 3: Evaporate to crystallisation point [1] [2] Step 4: L eave solution to cool [1]; dry crystals on filter paper [1] [2] [2] 3 a sulfur + oxygen → sulfur dioxide [1 for reactants; 1 for product] b SO2 is oxidised to SO3 and O3 is reduced to O2 c SO3 + H2O → H2SO4 [2] [2] [1] 4 a aqueous sodium chloride, copper, graphite [deduct 1 for each incorrect answer] b insulator [3] [1] anode [1] ii negative = zinc [1] ; positive = chlorine [1] [2] iii carbon [1] 5 a carbon/platinum [1] because unreactive [1] b bubbles [1] at both electrodes [1] c hydrogen at cathode [1], chlorine at anode [1] [2] [2] [2] Chapter C5 Acids, bases and salts 1 a pH 11 b slaked lime c i [1] [1] to help plants grow better (or words to that effect)[1] ii sulfur dioxide [1] from power stations [1] or nitrogen oxides [1] from car exhausts [1]; dissolves in rain [1] [3] d i neutralisation [1] ii Measure the calcium hydroxide/alkali with a pipette [1], add indicator [1] and add acid from burette until there is a colour change [1]. [3] 2 a pH 3 [1] b Add blue (or neutral) [1] litmus [1]; if it turns red, it is acidic [1]. [3] c i calcium carbonate + hydrochloric acid → calcium chloride + carbon dioxide + water [1 mark for each product] [3] ii in a blast furnace for producing iron 17 [1] [2] [1] ii Magnesium reduces zinc ions [1] by donating/ giving electrons to them [1] [2] c i Answers to end-of-chapter questions 4 a i ammonia gas is produced by the reaction [1]; ammonia turns moist red litmus blue [1] [2] ii no reaction if solid is ammonium nitrate [1] white precipitate if solid is ammonium sulfate [1] [2] b calcium carbonate reacts with acids [1]; calcium carbonate will neutralise acidic soil [1] [2] 5 a acidic: < 7, any appropriate e.g. SO2 basic: > 7, any appropriate e.g. CaO neutral: 7, any appropriate e.g. H2O [6] b i an oxide/substance that will react with/ dissolve in both acids and alkalis [1] ii any strong acid (e.g. HCl) + any strong alkali (e.g. NaOH) [2] Chapter C6 Quantitative chemistry 1 a b c d ammonia + sulfuric acid → ammonium sulfate 8 98 6.6 g [1] [1] [1] [2] 2 a 1.0 kg = 1000 g of conc. H2SO4 solution mass of H2SO4 = (1000 × 98) / 100 = 980 g (molar mass H2SO4 = 98 g/mol) number of moles H2SO4 = 980 / 98 = 10 moles [3] b i molar mass of CaO = 56 g/mol [1] number of moles CaO = 168 / 56 = 3 moles [1] [2] ii molar mass of H2O = 18 g/mol reacting ratio is 1 : 1 3 moles of water = 3 × 18 = 54 g [2] 3 40/1000 × 2 = 0.08 moles; 0.08/2 = 0.04 moles; 0.04 moles; 0.04 × 238 = 9.52 g [4] 0.08 moles; 6/119 = 0.05 moles [1] This is more than was necessary to react with all the HCl as 0.05 > 0.08/2 [1] [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences Chapter C7 How far? How fast? e i 1 The methods used to prevent explosions from ‘runaway reactions’ are precisely the opposite to those changes that would speed up the rate of reaction. The changes used would need to have a ‘dampening’, or inhibitory, effect. The following changes would all result in a slowing down of a reaction: • lowering the temperature • adding water to dilute the reactants (see Workbook Exercise 7.6 for an example) • lowering the pressure of a gas reaction. 2 a hydrated iron sulfate → anhydrous iron sulfate + water [1] b endothermic [1]; heat has to be applied (or words to that effect) [1] [2] c pale green [1] d It is a reversible reaction [1], hydrated iron sulfate is formed [1] and heat is also generated (reaction exothermic) which produces steam [1]. [3] e reversible reaction [1] f If water is added to anhydrous cobalt chloride [1], it changes colour from blue to pink [1]. [2] 3 a Carbon dioxide is given off. somewhere between 600 and 630 s respiration [1] ii a substance which speeds up a chemical reaction [1] 4 a amount of manganese(iv) oxide and temperature [2] b i the higher the concentration, the faster the reaction [1] ii A lower concentration will produce less oxygen. [1] iii 25 or 26 s [1] 36 or 37 cm3[1] c magnesium oxide, copper(ii) oxide, manganese(iv) oxide, lead(iv) oxide [1] 5 a i Other methods are also possible. b i Answers to end-of-chapter questions fair test [1]; keep the amount of solution above the cross the same [1] [2] ii value for gap: between 120 and 150 [1] iii speed decreases [1] because lower concentration [1] means fewer collisions [1] [3] b The reaction is faster [1] because higher temperature makes particles move more rapidly [1]; this means more collisions [1] and harder/more energetic collisions [1]. [4] 6 a temperature, surface area of magnesium; [1] (length, mass or size of magnesium (ribbon) would be allowed, simply writing ‘amount of magnesium’ not allowed) [1] b i [1] B the graph shows a higher rate / is steeper;[1] ii X placed at the beginning of the curve (see graph below) [1] ii (maximum volume of gas) 40 cm3 at reaction time of 5 minutes / 300 s (1); iii sketch graph to the right of the printed curve [1] and levelling out above it [1] [2] average rate = 40 ÷ 5 = 8 / 40 ÷ 300 = 0.13 (1); units: cm3 / minute or cm3 / s (1) c i Mass of flask and contents / grams 100.4 in aqueous (solution) / dissolved in water / in solution;[1] 100.3 ii same mass / length / size / amount of magnesium used in both experiments (1); 100.2 acid in excess / all magnesium used up in both (1); gas volume produced depends on amount of magnesium used (1) [2] 100.1 100.0 0 c i Chapter C8 Patterns and properties of metals 100 200 300 400 500 Time / seconds 600 700 gets faster [1] ii gets faster d combustion, small, large [1] [3] 18 [3] 1 a Alloys are metals whose composition is designed to suit the properties required by a particular use or situation. Properties which have been significant in the development of alloys have included: • tensile strength • hardness © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences • • • • resistance to corrosion Chapter C9 Industrial inorganic chemistry electrical conductivity 1 Although there are some cases where recycling has significant economic advantages in terms of costs (for example, the recycling of aluminium), this is not always the case. The most important argument for recycling is the conservation of natural resources, particularly nonrenewable resources of minerals and fuels, for instance. low melting point colour. b Brass is used in plugs and switches because, even though it is not as good a conductor as copper, it is cheaper and harder. It is more resistant to hard wear and regular use. 2 a i copper, zinc, magnesium, calcium [1] ii Iron does not react with cold water [1] but it does react with steam when heated [1]. [2] b zinc + water → zinc oxide + hydrogen [1] c high melting point/boiling point, malleable, conduct heat, conduct electricity (any three) [3] d i any sensible answer above 98 °C ii decreases [1] [1] iii any sensible answer above 0.53 g/cm3 and below 1.0 g/cm3 (it floats on water) [1] 3 a i lithium + water → lithium hydroxide + hydrogen [2] [1] ii 2Na + 2H2O → 2NaOH + H2 b lithium reaction not exothermic enough to melt the metal, sodium and potassium melt into a ball, potassium ignites spontaneously order of increasing reactivity Li<Na<K all float on water, all fizz and produce hydrogen, all leave an alkaline solution (any five points) [5] c i anode: E [1]; electrolyte: A [1] [2] ii positive = chlorine; negative = sodium [2] iii graphite d low melting point, soft/can be cut with knife, electrical conductivity, etc. (any two) [1] [2] 4 a i Mg + 2HCl → (MgCl2) + H2 formulae (1); balancing (1) [2] ii magnesium > X > copper [1] b i solution turns blue to colourless / becomes fainter (1); brown deposit (of copper) (on metal X) (1) [2] ii X is less reactive than magnesium / magnesium is more reactive than X [1] c i removal of oxygen / gain of electrons [1] ii metal ions have a positive charge (1); cathode has a negative charge and opposite charges attract (1) [2] 19 Answers to end-of-chapter questions The impact of efficient recycling can be wide-ranging. The demand for rare metals for the electronics and media industries puts great pressure on the need to find new mineral resources and the development of new mining ventures. This can bring conflicts with environmental concerns in some of the most untouched areas of the world. Efficient recycling could delay some of these potential clashes of interest. 2 a acidic soil [1] b nitrogen [1] c ammonium sulfate + calcium hydroxide → ammonia + water + calcium sulfate products or ‘double decomposition’[2] d CaCO3[1] e CaCO3 → CaO + CO2[2] f water is added [1] g N2 + 3H2 ⇌ 2NH3; high pressure, moderate temperature (or values), catalyst [4] h 2SO2 + O2 ⇌ 2SO3; moderate temperature, catalyst [4] 3 a A: yes will rust, has air and water [1]; B: no, has air but no water [1]; C: no, has air and water but protected/coated with zinc [1] [3] b carbon burned off by oxygen as carbon dioxide [1]; phosphorus, etc., react with calcium oxide/lime to form slag [2] [3] c surgical instruments, chemical plant, cutlery (any of these) [1] 4 redox reaction: Fe2O3 + 3CO → 2Fe + 3CO2 (or give the equation with carbon) acid/base reaction: CaO + SiO2 → CaSiO3 Carbon burns to give heat and form carbon dioxide. Carbon dioxide reacts with carbon to form carbon monoxide. Carbon monoxide reduces hematite to iron. Limestone decomposes to calcium oxide and carbon dioxide. © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences • the synthetic and natural polymers that provide us Calcium oxide (lime) reacts with silica to form slag. (two equations plus three other points of description) [5] 5 a i oxygen = top left [1] ii slag = right [1] iii molten steel = bottom left [1] b i They are gases. [1] ii They react together to form calcium phosphate, which is a solid, and form slag, which floats on the steel. [3] c i D ii surgical instruments, chemical plant, cutlery (any of these) [1] [1] 6 a decomposition [1] b so that the ions are free to move [1] c to lower the operating temperature by lowering the melting point of the electrolyte [1] d B [1] e anode = oxygen or carbon dioxide [1]; cathode = aluminium [1] [2] f because they burn away [1] in the oxygen [1] [2] 3+ – g Al + 3e → Al [1] h pans, cans, power cables, aircraft bodies, etc. [1] 7 a i reduction Answers to end-of-chapter questions [1] ii carbon monoxide [1] iii coke (carbon) and hot air [1] b copper is less reactive than iron / bonding in copper oxide is weaker (1); less energy needed to break bonds (1) [2] c limestone is added to the blast furnace (1); limestone decomposes in the furnace to give calcium oxide / lime (1); silicon dioxide reacts with calcium oxide to produce slag / calcium silicate (1) [3] with food, clothing and structural materials that support our living and the technologies we depend on • the novel structures that provide the scope for developing nanotechnology. 2 a i hydrogen and carbon contain just one type of atom; compounds contain different atoms bonded together elements are listed on the Periodic Table; compounds are not [2] ii draw a central C with four hydrogens attached by single bonds [2] iii natural gas b i [1] Z[1] ii X, Z unsaturated molecules contain double bonds [2] iii pass the gas through bromine water [1]; an unsaturated hydrocarbon will decolorise the bromine water [1], a saturated hydrocarbon will not [1] [3] 3 a a family of organic compounds with similar chemical properties due to the presence of the same functional group [1] b A = alkene; B = alkane; C = alcohol [3] c test: bromine water; A: decolorises; B: no effect [3] d heat it with steam [1] and a catalyst [1] [2] e fermentation [1] 4 a ethane (1); C2H6 (1) b i [2] a homologous series [1] ii methane (1); CH4 (1) [2] iii Chapter C10 Organic chemistry 1 The versatility of carbon lies in its ability to form chain and ring structures, and to form multiple bonds with itself and other atoms. The complexity that arises is important to us in several different ways: • the chemistry of life and the interactions between carbon-containing molecules that generate the energy for living cells and the way of passing genetic information from one generation to the next • the carbon-containing compounds – from fossil [2] c the boiling point of the alkanes increases with the size of the molecule (1); the larger (longer) the molecule the greater the forces (interactions / inter-molecular forces) between the molecules (1); more energy is needed to disrupt/break/overcome these forces (1) [3] fuels – that are the fuels of our modern transport and energy-generating systems 20 © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences d i 3 a i CH4 + 2O2 → CO2 + 2H2O formulae (1); balancing (1) [2] ii number of moles carbon dioxide = 480 / 24000 (1) = 0.02 mol (1) [2] iii from equation: number of moles of methane used = 0.02 mol (1) Mr of methane = 16 (1) mass of methane burnt = 0.02 x 16 = 0.32 g (1) [3] 1 The products of burning methane, ethanol and fuels such as gasoline are the same – the question is more one of the efficiency and our ability to use the fuels cleanly. Methane and ethanol are single compounds but gasoline is a mixture of hydrocarbons and more difficult to burn completely. Incomplete combustion gives rise to pollution with carbon monoxide, soot and particulates. Ethanol is more environmentally friendly because it is/ can be a renewable fuel. Any carbon dioxide released can be at least partially removed from the atmosphere as (for example) the sugar cane used in fermentation is grown. Methane is more environmentally friendly as it produces less carbon dioxide for the amount of energy it releases as it has the best/highest carbon : hydrogen ratio. 2 a i hydrogen and carbon (each) contains one type of atom / is found in the Periodic Table / cannot be broken down into simpler substances; propane contains different atoms (or elements) bonded together / can be broken down into simpler substances / into elements [2] ii petroleum / natural gas [1] iii fractional distillation [1] iv heating / lighting / burners / cooking / vehicle fuel / refrigerant / feedstock [1] (catalytic) cracking [1] ii only single bonds (in a molecule) / contains maximum possible hydrogen atoms [1] iii ethene and propene [1] iv structure of ethene: draw two C linked by a double bond, four H attached by single bonds (two to each C) [2] 21 structure of ethene: draw two C linked by a double bond, four H attached by single bonds (two to each C) [2] ii molecule contains at least one C=C double bond; does not contain the maximum possible number hydrogen atoms [1] b i many ethene molecules join together to make a long chain; draw a series of units joined together [2] Chapter C11 Petrochemicals and polymers b i Answers to end-of-chapter questions ii addition polymerisation [2] 4 a boiling point [1] b fuel oil – fuel for home heating; kerosene – jet fuel; lubricating fraction – waxes and polishes; naphtha – making chemicals [4] c i heat and catalyst [2] ii C14H30 → C2H4 + C12H26 iii H [1] H C C [1] H H d poly(ethene) [1] e i [1] steam ii a substance that speeds up a reaction 5 a i a group of hydrocarbons with boiling points close together [1] [1] ii C12H26 → C2H4 + C10H22 [1] b heating and cooking; fuel for cars [2] c molecules contain a double bond; a compound of carbon and hydrogen only [2] d i catalytic addition of steam ii H H H C C H H [1] O H [2] e monomers, polymers [2] 6 a condensation polymer [1] b i 20 [1] ii Mr = (1 x 16) + (14 x 2) + (12 x 6) = 116 [2] c (at least) three monomer units shown (1); correct sequence (1); amide links shown (1) [3] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences d it (is a covalent substance) does not conduct electricity[1] f i f correct plotting [2]; two straight lines [1] [1] it is a gas which absorbs heat (1); retains heat in the atmosphere / raises the temperature of the air (1) [2] ii by catalysing a reaction between nitrogen dioxide and carbon monoxide (1); to produce carbon dioxide and nitrogen (1) [2] [3] 1.20 1.00 Total increase in mass / g e the molecules are not all the same length / it is a mixture of molecules (of different lengths) Answers to end-of-chapter questions 0.80 0.60 0.40 0.20 Chapter C12 Chemical analysis and investigation 0.00 2 a arrow under copper oxide b black [1] to orange/brown/pink [1] c diagram of condenser tube through [1] cooling jacket [1] d extinguished [1] [2] 3 a carbon or platinum b cathode (negative electrode) c bubbles [1] [1] [1] d i with an organic solvent – ethanol or propanone ii using a hair dryer e 0.75, 1.00, 1.15, 1.15, 1.15 22 [2] [1] 10 20 30 40 Time / min 50 60 70 g reaction finished/copper sulfate gone, current switched off (any of these) [1] 4 a initial: 25, 26, 23, 24 final: 28, 39, 46, 58 rise: 3,13, 23, 34 [–1 for each incorrect value] [4] b correct plotting [3] [–1 for each error], straight line [1] (see graph below) [4] c extrapolation of line to 5 [1], correct reading of temperature (44 °C) from extrapolation [1], units [1] (see graph below) [3] 50 40 Temperature rise / °C 1 Analysis of the substances we discover and use in the wide variety of activities that shape our lives is important in terms of our control of our environment, the efficient use of resources available to us and our protection from the harmful effects of contamination and misuse. Chemical analysis can be used in industry, medicine, agriculture and environmental science. We need to know what chemicals we are dealing with. Analytical techniques, from the simple to the complex, help us to do just that. A medical drug company might need to analyse the painkiller paracetamol, for instance, in order to maintain the purity of the product it is marketing. A steel-making company must check if the content of a batch of steel matched the customers requested composition. An environmental analyst might have to check river water for contamination with small amounts of metal ions, which may be harmful to local wildlife and also in the drinking water supply. 0 30 20 10 0 5 1 2 3 4 Number of carbon atoms in the alcohol formula d temperatures would be higher [1], because copper is a good conductor [1] [2] [1] [1] [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 5 a chromatography [1] b line below origin [1] c inks colours would interfere with the result (or words to that effect) [1] d difference: A had more colours that B [1]; similarity: both contained colour E [1] [2] e C,D and E [1] 23 Answers to end-of-chapter questions 6 a, b Provided in question as example answers. c i white precipitate that dissolves in excess [3] ii white precipitate, insoluble in excess d solid contained water (of crystallisation) e ammonia f E is not a sulfate [1], contains nitrate ions [1] [2] [1] [1] [2] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences Physics mass c density = volume [1] 102 = 25 1 a volume = l × b × h[1] = 8.4 × 8.0 × 5.5 [1] = 369.6 cm3[1] [1] = 4.08 g/cm3[1] d Sample m / g V2 V1 V / cm3 Density / 3 3 [1] / cm [1] / cm [1] g/cm3 [1] [1] [1] mass b density = volume [1] 340 = 369.6 [1] = 0.92 g/cm3[1] 2 mass of liquid = 203 – 147 [1] [1] = 56 g density = mass volume [1] = 56 59 [1] = 0.95 g/cm3[1] 3 a volume = l × b × h[1] = 80 × 40 × 15 [1] = 48 000 m3[1] b mass = volume × density [1] = 48 000 × 1.3 [1] = 62 400 kg [1] 4 a Half-fill a measuring cylinder with water; record volume.[1] Place pebble in water, ensuring that it is submerged.[1] Record new volume. [1] Volume of pebble equals difference in recorded volumes.[1] b mass of pebble [1] 5 a 30.98 − 30.72 = 0.26 g mass b density = volume [1] [1] 0.26 = 200 [1] = 0.0013 [1] g/cm [1] 3 6 a V1 = 70 cm [1] 3 V2 = 95 cm3[1] b V = 95 – 70 [1] = 25 cm3[1] 24 7 a b c d e B 144 80 44 36 [1] 4.0 [2] C 166 124 71 53 [1] 3.1 [2] water volume (of water) or water level the stone volume (of water) subtract or calculate the difference between first volume from (or and) second volume [1] [1] [1] [1] [1] [1] Chapter P2 Describing motion distance 1 average speed = time [1] = 400 50 [1] = 8.0 m/s [1] 2 distance = speed × time [1] = 15 × 30 [1] = 450 m [1] 3 a speed of light b distance = speed × time [1] [1] 4 Speed is uniform (constant) in both. [1] The bus travels faster during B than A. 5 a [1] 800 Distance / m Chapter P1 Making measurements Answers to end-of-chapter questions 600 400 200 0 0 10 20 30 Time / s 40 suitable scales chosen [1] horizontal axis and scale correct [1] vertical axis and scale correct [1] five points correctly plotted and straight line drawn[1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences b Graph is straight line, [1] so constant speed. [1] 6 speed is constant [1] acceleration = 0 [1] Speed 7 a Time distance travelled [1] 12 a average speed = time taken b m/s [1] cGraph is a horizontal straight line, showing that speed does not change. [1] d distance travelled = area under graph [1] 13 a 25 km [1] b i accelerating or increasing speed ii steady or constant speed iii decelerating or slowing down c less than [1] [1] [1] [1] 14 a [1] [1] [1] 30 Speed / m/s horizontal axis showing time vertical axis showing speed rising straight-line graph starting at origin Answers to end-of-chapter questions b 20 10 Speed 0 horizontal straight-line graph above axis, then decreases down to zero [1] [1] 8 a B, D b A, E c Acceleration is changing in the other section, C. [2] [2] [2] change in velocity 9 acceleration = time taken [1] 8.0 = 2.0 [1] = 4 m/s2[1] 10 initial speed = 0 m/s change in speed = acceleration × time [1] [1] = 2.3 × 4.0 [1] = 9.2 m/s speed 11 time = acceleration [1] [1] 24 = 5.6 [1] = 4.3 s 25 [1] 10 20 30 Time / s 40 50 horizontal axis and scale correct vertical axis and scale correct six points correctly plotted graph drawn through points [1] [1] [1] [1] b acceleration = gradient of graph [1] Time horizontal and vertical axes showing time and speed[1] 0 27 = 30 [1] = 0.9 m/s2[1] c distance = area under speed against time graph [1] = area of triangle + area of rectangle [1] 1 = 2 × 30 × 27 + 20 × 27[1] = 405 + 540 [1] = 945 m [1] 15 a iconstant/steady/uniform speed or velocity or speed or velocity = 2.5 (m/s) [1] speed or velocity = 2.5 m/s ii shape curving upward but not to vertical [1] [1] bhorizontal (straight) line (parallel to time / x-axis)[1] c i horizontal straight line at 2.5 m/s from 0 to 2 s [1] ii straight line rising to the right as far as the edge of the graph area [1] Δv = 4 m/s or gradient clearly 2 m/s2[1] dhorizontal straight line [1] at 0 m/s [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences Chapter P3 Forces and motion 1 resultant force [1] 2 a weight b friction [1] [1] 3 a weight downwards, air resistance upwards [2] b zero [1] c The resultant force on it is zero, so it does not accelerate.[2] 4 a resultant force = 680 – 600 = 80 N upwards b He will accelerate upwards. [1] [1] [1] [1] 5 a weight = mass × g [1] = 80 × 10 = 800 N b the same c less [1] [1] [1] [1] 6 a force = mass × acceleration [1] b kilogram (kg) or gram (g); newton (N); metre per second per second (m/s2)[3] 7 the bigger force acting on the smaller mass, [1] that is, the 10 N force acting on the 5 kg mass [1] 8 force = mass × acceleration [1] = 20 × 5 [1] = 100 N [1] force [1] 9 acceleration = mass 1400 000 = 800 000 [1] = 1.75 m/s2 [1] change in speed [1] time (20 – 12) = 6.4 [1] 10 acceleration = Answers to end-of-chapter questions 12 a the two 5000 N forces [1] They are equal in size but act in opposite directions. [1] b resultant force = 1300 – 1200 = 100 N [1] [1] forwards (to the left) [1] c The lorry will speed up (accelerate). [1] 13 a i (engine) thrust and (air) friction [1] ii force shown vertically upwards, anywhere on plane[1] distance b i speed = time in any form [1] 2200 = 2.75 [1] = 800 (km/h) [1] ii idea of headwind on outward journey or tailwind on return journey or routes of different lengths or less friction or less weight [1] (v–u) or v or 8 14 a i 3 [1] t t = 2.7 m/s2[1] ii F = ma or 42 × 8/3 [1] = 112 N [1] iii distance in first 3 s = 12 m [1] so distance in last 11.2 s = 88 m [1] 88 so final speed = 11.2 = 7.9m/s[1] b Any two from: lower top speed, longer total time, less steep slope at first, etc. [2] Chapter P4 Turning effects of forces 1 moment [1] 2 a resultant b zero [1] [1] 3 a, b for example [3] = 1.25 m/s2[1] force = mass × acceleration [1] = 1200 × 1.25 = 1500 N [1] [1] 11 weight = mass × g [1] = 50 × 1.6 = 80 N [1] [1] 26 centre of mass centre of mass stable object unstable object © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 4 a x pivot F b moment = force × distance from pivot c Quantity Unit force N distance m moment of force Nm [1] [1] [3] 5 See Activity P4.03. Make three small pinholes around the edge of the lamina.[1] Suspend the lamina freely from a pin through one hole. [1] Mark a vertical line below the pin using a plumb-line.[1] Repeat this process for the other two pinholes. [1] The centre of mass is where the three lines intersect.[1] 6 contact force A 1m 0.9 m centre of mass B 1.5 m weight of beam a centre of mass correctly marked, as in diagram b arrows and labels added correctly c moment of weight = force × distance = 200 N × 0.5 m = 100 N m moment of force F is F × 1.0 = 100 N m so F = 100 N d upward contact force = sum of downward forces = 200 N + 100 N = 300 N 7 a force and perpendicular distance (of force) from the point b i 27 downward force arrow at centre of bar [1] [2] [1] [1] [1] [1] [1] [1] [1] [1] [1] [1] Answers to end-of-chapter questions ii 0.50 m or 50 cm [1] iii moment of force = 40 × 1.2 = 48 N m [1] moment of weight = + 30 × 0.5 = 15 N m [1] total clockwise moment = 48 + 15 = 63 N m [1] iv F × 0.2 = 63 [1] 63 F = = 315 N[1] 0.2 v make bar longer or move pivot/stone to the left or move pivot to left or increase mass of bar [1] Chapter P5 Forces and matter 1 a increases b decreases [1] [1] 2 If you stand upright, your weight is pressing down on a small area. [1] This gives a high pressure. [1] If you use a ladder, the pressure is less because your weight is spread over a greater area. [1] 3 a extension = length when stretched – original length[1] b graph b[1] 4 a The extension of a spring is proportional to the load, provided the limit of proportionality is not exceeded.[2] b load = stiffness × extension [2] c See Figure P5.05a. [3] 5 extension = change in length = 66 – 58 = 8.0 cm [1] [1] [1] 6 extension for 5 N is 15 – 12 = 3.0 cm [1] extension for 15 N is 3 × 3 cm = 9.0 cm [1] length is 12 + 9 = 21 cm [1] 7 See Activity P5.01. Diagram or list indicating: • spring hanging vertically from clamp [1] • weights hanging from end of spring [1] • ruler[1] Student must measure: • length of spring when weights added • unstretched length of spring • repeated for at least five different weights. [1] [1] [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences b Load / N Length / cm Extension / cm 0.0 83.0 0.0 5.0 87.0 4.0 [1] 10.0 91.0 8.0 [1] 15.0 95.0 12.0 [1] 20.0 99.0 16.0 [1] Extension / cm 2.0 60 40 20 0 0 20 1.0 0.5 0 5 10 Load / N 15 20 horizontal axis and scale correct [1] vertical axis and scale correct [1] five points correctly plotted and graph drawn through points [1] force 9 a pressure = [1] area F b P = [1] A [3] c Quantity Unit 28 80 1.5 0 10 a b Extension / mm 8 a Answers to end-of-chapter questions force N area m2 pressure Pa 40 Load / N 60 80 horizontal axis and scale correct [1] vertical axis and scale correct [1] eight points correctly plotted [1] graph drawn through points [1] c Draw up from 25 N to intersect graph line [1] from this intersection, go across to axis, 19 mm [1] d the point where the graph line ceases to be straight[1] 40 N approximately [1] 11 aextension indicated between two broken lines[1] b i four points correctly plotted [2] straight line through points and origin [1] ii proportional [1] iii 1 newton(s) [1] 2 extension = 25 − 26 mm length = 75 − 76 mm Load / N Length / cm Extension / cm 0 3.200 0 10 3.207 7 b i two from 20 3.215 15 30 3.222 22 40 3.230 30 50 3.242 42 • • • • 60 3.255 55 70 3.270 70 12 a wall A has bigger area so lower pressure (on soil) [1] [1] [1] [1] depth/height of air/atmosphere density of air/atmosphere acceleration due to gravity or weight of air of air [2] ii 1 the same as [1] 2 greater than or four times [2] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences Chapter P6 Energy transformations and energy transfers 1 Answers to end-of-chapter questions Name Description kinetic energy energy of a moving object useful energy output[2] × 100 % b efficiency = energy input 1 2 8 a k.e. = 2 mv (m = mass, v = speed)[3] bg.p.e. = mgh (m = mass, g = acceleration due to gravity, h = height) [4] internal energy energy stored in a hot object 9 work chemical energy energy stored in a fuel potential / gravitational / p.e. / g.p.e. / position [1] light energy energy that we can see kinetic / k.e. / movement [1] sound energy energy that we can hear constant / the same / uniform [1] strain (elastic) energy energy stored in a squashed spring joule(s) or J [1] electrical energy energy carried by an electric current nuclear energy energy stored in the nucleus of an atom heat thermal energy energy escaping from a hot object 2 a chemical energy → light + heat 10 a weight = mass × g[1] = 180 × 1.6 [1] = 288 N [1] b change in g.p.e. = weight × change in height = 288 × 100 = 28 800 J [1] [1] [1] [9] c g.p.e. increases [1] [2] 11 a mgh = 0.5 × 10 × 1.1 = 5.5 J [1] [1] b electrical energy → kinetic energy [2] c kinetic energy → electrical energy [2] d kinetic energy → thermal (heat) energy [2] 3 energy supplied = 100 J energy released = 93 + 7 = 100 J [1] [1] Energy before is equal to energy after, so energy is conserved.[1] 4 a gravitational potential energy → kinetic energy [2] b kinetic energy → gravitational potential energy [2] cSome energy is lost as heat due to friction and/or air resistance, [1] b i 1.5 (J) c 9 + 5.5 = 14.5 J [1] 1 2 k.e. = 2 mv [1] v = 7.6 m/s [1] Chapter P7 Energy resources [1] 5 a heat energy [1] 2 a Trees and plants grow b efficiency [1] c conservation [1] so the final g.p.e. cannot equal the original g.p.e. [1] by jumping up as she starts off. 6 a thermal (heat) energy, electrical energy [1] [2] b thermal (heat) energy [1] c Yes, because 90% of the energy is used, [1] and only 10% is wasted. [1] 7 awaste energy = energy input – useful energy output[2] 29 [1] iienergy used to deform ball/ground or strain energy stored in (deformed) ball/ground or heat generated in deformed ball/ground [1] 1 a b c d e d She needs to supply energy, [1] resource Sun renewable fossil fuels; non-renewable wind, electricity [1] [1] [1] [2] [2] [1] using sunlight as their energy source. [1] b Sunlight causes evaporation, producing clouds;[1] rain falls, and finally enters rivers, [1] whose water is trapped behind a dam. [1] 3 a g.p.e. b k.e. c g.p.e. → k.e. → electrical energy [1] [1] [2] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 4 a i Answers to end-of-chapter questions fission [1] 4 Ahmed [1] ii uranium [1] He lifts them to a greater height. 5 a Millie: speed = 25 = 0.50 m/s 50 100 = 0.40 m/s Lily: speed = 250 b Millie [1] b i fusion [1] ii hydrogen [1] iii helium [1] 5 a Sunlight is always available in space, and not much power is needed on a spacecraft. [1] But in cities, there are large numbers of people in a small area, [1] so there is not enough roof space for all the solar cells that would be needed to generate enough power.[1] b (for example) In a desert for roadside phones, because there is then no need to connect the phone to the mains electricity supply. c A rechargeable battery can store the energy produced by solar cells, Because they are identical, the one with the greater speed has the greater power. [1] 7 a oil nuclear fission b i gas lamp [1] ii electric motor or loudspeaker [1] iii microphone [1] Chapter P8 Work and power 1 a more b more [1] [1] 2 a energy b work [1] [1] 3 a work done = force × distance moved (in the direction of the force) [2] b [3] Quality Unit W joule, J F newton, N x 30 metre, m [1] [1] [1] = 700 × 2.5 = 1750 J [1] [1] gravitational potential energy (g.p.e.) [1] iiforce/mass/weight of (basket of) rocks [1] [1] 9 [1] [1] = 250 × 12.0 = 3000 J b gain in g.p.e. = weight × increase in height 8 a i [2] [At least two correct in each column for 4 marks; deduct 1 mark for any in incorrect column.] [1] 7 a work done = force × distance moved 6 Renewable: two from hydroelectricity, solar, tidal, wind[2] Non-renewable: two from coal, oil, nuclear [1] [1] [1] and can therefore supply electricity when the sun is not shining. [1] [1] 6 work done = energy transferred [1] [1] [1] and height of cliff b chemical energy [1] c time taken to raise basket up cliff [1] [1] a weight = mass × g[1] = 45 × 10 = 450 N [1] b gain in g.p.e. = weight × increase in height = 450 × 0.20 × 36 [1] [1] = 3240 J [1] work done [1] c power = time taken 3240 = 4.2 [1] = 770 W [1] = 0.77 kW [1] 10 a work done = force × distance moved [1] = 780 × 100 [1] = 78 000 J [1] b work done = force × distance moved [1] = 240 × 100 [1] = 24 000 J [1] [9] 1 2 c k.e = × 750 × 12 [1] = 54 000 J 2 [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences dwork done by engine = work done against friction + k.e. [1] so energy is conserved [1] 11 a M = V × D = 103 × 10-3[1] = 1.0 kg [1] b mgh = 1 × 10 × 0.8 [1] = 8.0 J (or 8.0 N m) E 8 × 90 c P= = t 60 = 12 W (or 12 J/s or 12 N m/s) [1] so the average energy of the molecules remaining decreases. [1] [1] Hence its temperature decreases. [1] 1 See Figure P9.01 in the Coursebook. [6] 2 See Figure P9.03 in the Coursebook. [3] 3 a evaporation b faster-moving or more energetic; decrease or fall/drop [1] [2] 4 a gas b solid c liquid [1] [1] [1] 5 a solid b The particles are well separated and can move about within the volume of their container, [1] colliding with its walls and with each other. [1] [1] [1] b temperature [1] 7 a smoke particles b molecules of the air [1] [1] 8 a particles of smoke [1] b The smoke particles are moving because the particles of the air are continually colliding with them,[1] [1] 9 a slowly [1] b quickly [1] c quickly [1] bIn the liquid, forces between the particles hold them together. 31 [1] [1] Chapter P9 The kinetic model of matter 10 a evaporation (or vaporisation) so that its mass decreases. [1] 8000 Pa (or 8000 N/m2)[1] changing their speed and direction of motion. 11 aMolecules of ethanol leave the surface of the liquid[1] bThe more energetic molecules of ethanol are more likely to leave the liquid, d P = ρgh[1] 6 a energy If it is to become a gas, energy must be supplied to overcome these forces and separate the particles.[1] [1] 78 000 = 24 000 + 54 000 Answers to end-of-chapter questions [1] [1] 12 Shape Molecules a Solid fixed shape [1] vibrate about a fixed position b Liquid shape fills the container from the bottom [1] move around, close together c Gas completely fills the container move around, far apart [1] [1] 13 a ibombardment/collisions with air molecules/ particles[1] ii any two from lighter / very small / smaller than smoke particles / too small to be seen fast-moving / high kinetic energy random movement / movement in all directions [2] b i increases [1] iiair molecules/particles/atoms bombard/ hit walls [1] molecules faster / higher energy when temperature raised [1] (not vibrate faster) greater force (per unit area) or more collisions per second [1] Chapter P10 Thermal properties of matter 1 a See examples in Section P10.01 of the Coursebook. [1] b See examples in Section P10.01 of the Coursebook. [1] 2 a Liquid in bulb absorbs energy; gets hotter; expands; pushes up tube. [3] b melting point of pure ice (0 °C); boiling point of pure water (100 °C) [2] 3 a Mercury expands as its temperature increases. [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences b Definition Value lower fixed point melting point of pure ice 0 °C upper fixed point boiling point of pure water [1] 100 °C b Electrons collide with particles in hotter region, gain energy; move randomly to cooler region, collide with particles there, give them energy. [3] [1] c (for example) the resistance of a resistor or thermistor 4 expands; greater; less; lighter; rises; more; gravity; convection[8] 5 [1] 4 a A has greater range (120 °C, from –10 °C to +110 °C). (B’s range is only 60 °C, from –10 °C to +50 °C.) [2] b B is more sensitive. Each degree is a wider interval on the scale, so smaller changes can be measured. [2] 5 solids, liquids, gases[3] 6 a the thermocouple thermometer b 100 °C [1] [1] This is a fixed point on the Celsius scale. c the liquid-in-glass thermometer [1] [1] It can measure to 0.5 °C (or better); the other measures to the nearest 1 °C. [1] d The properties of the two materials used in the thermometers do not vary linearly with temperature.[1] The voltage of the thermocouple does not increase at a steady rate as the temperature goes up. 7 a 0 and 100 (°C) b i [1] [1] expands [1] ii moves along the tube/up/to the right [1] stops at/near 100 mark [1] c arrow slightly to left of –10 mark b metal; non-metal 2 convection Warm fluid moves, carrying energy with it. radiation Energy travels as infrared waves. conduction Energy travels through a material without the material moving. Good emitter Good reflector matt [1] matt [1] shiny [1] black [1] black [1] white [1] 6 a As the air is heated, it expands. [1] Its density decreases. [1] It is lighter than the surrounding air, so it floats upwards.[1] b The surrounding air is cooler and so less dense. [1] It sinks and replaces the warm air rising above the flame.[1] 7 a Particles at the hot end have greater energy, so vibrate more. [1] They collide with neighbours, sharing energy with them.[1] Energy is thus transferred from the hot end to the cold end. [1] b The temperature of the cold end of the rod would rise more rapidly, [1] because metals are better conductors than plastics.[1] c electrons [1] 8 a walls made of glass – poor conductor [2] [1] [3] lid – prevents convection losses (but see part b)[2] [2] silvering – reflects away infrared radiation [2] bA liquid that is colder than its surroundings does not heat the air above it, [1] so no convection current rises above it. Hence a lid is not essential. [1] 9 aAir is a good insulator, so less heat is lost by conduction.[1] [2] 3 a Particles in hotter region vibrate more; collide with cooler neighbours and share energy; these vibrate more, pass energy on to their neighbours; and so on. [3] 32 Good absorber vacuum between walls – no conduction or convection[2] Chapter P11 Thermal (heat) energy transfers 1 a temperature; higher; lower Answers to end-of-chapter questions Cold air from the window cannot flow into the room, so convection current losses are reduced. [1] bInfrared (heat) radiation from below is reflected back into the house, so that less escapes from the house. [1] [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences cThe glass wool prevents the movement of air in the gap, so it is difficult for a convection current to be set up, [1] c The vibrations of the instrument cause the air near the instrument to vibrate. [1] which would transfer energy from the inner wall to the outer wall. [1] 10 a i conduction [1] ii convection [1] b heat lost at same rate as heat supplied [1] c i boiling [1] ii steam [1] 11 a i conduction [1] [1] [1] [1] [1] The frequency of trace C is the greatest (because more waves are contained in the same time interval). [1] 7 You need a source of sound, and two detectors in line with the sound. [1] [1] You need to measure the distance between the two detectors,[1] and the time interval between the sound reaching them.[1] distance Then use speed = time to calculate the speed of sound. [1] 8 Chapter P12 Sound 1 a source and these propagate through the air to the listener’s ear. b trace C [1] [1] [1] [1] [1] [1] The amplitude of trace A is the greatest. bcopper is a better conductor or iron is a worse conductor[1] c iron conducts heat slowly so gas above gauze is hot enough to burn copper conducts heat rapidly so gas above gauze is not hot enough to burn Compressions and rarefactions are formed, 6 a trace A iiatoms/free electrons at hot end vibrate more/ have more energy [1] share energy with others by collisions Answers to end-of-chapter questions rarefaction where particles of the medium are spread out compression where particles of the medium are squashed together [1] [1] b vibrations [1] c echo [1] d frequency; second [2] tap table at a distance [1] e hertz [1] and hear the sound through the wood. [1] f [2] c distance travelled = 2 times length of rod 2 a greater frequency [2] b greater amplitude [2] gases; vacuum 3 a B A [2] b D [2] C 9 a solid [1] b (for example) Place ear against table, = 800 m [1] distance [1] time 800 m = 0.16 s [1] = 5000 m/s [1] speed = 10 a i reflection or wave bounces back [1] from large object/sea bed [1] ii distance = speed × time [1] = 1500 × 0.80 [1] = 1200 (m) [1] iii 1200/2 = 600 (m) [1] b graph should show 4 shaded from 20 Hz to 20 kHz [2] 5 a the air inside the instrument [1] uniformly sloping line [1] b the strings of the instrument [1] with positive gradient [1] 33 © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 11 a any large surface, e.g. wall / cliff / mountain b i when hears bang / sees flash [1] Two rays in different directions from a single point on the lamp [1] reflect off the mirror correctly [1] and are extrapolated back behind the mirror, [1] so that the image is at the point where they cross. [1] [1] ii when hears echo [1] c i reading = 2.25 s [1] distance [1] time 720 = [1] 2.25 = 320 (m/s) [1] speed = Answers to end-of-chapter questions ii one from b Each ray is reflected [1] so that angle of incidence equals angle of reflection.[1] 6 a ray 2 inaccurate distance from firework I F ray 1 F reaction time wind [1] Ray 1 continues straight through the centre of the lens,[1] ray 2 bends at the lens [1] and passes through the principal focus F, [1] so that the image is at the point where they cross. [1] Chapter P13 Light 1 a See Figure P13.01 in the Coursebook. [4] b angle of incidence = angle of reflection i = r[2] 2 a virtual b The image is diminished [1] because it is shorter than the object. b the same size as [1] c object [1] d left–right inverted [1] 7 See Figure P13.03a in the Coursebook. [2] 8 3 See Figure P13.08a in the Coursebook. 4 O incident ray [1] c The image is inverted because it is below the axis. [1] normal mirror angle of incidence i [1] [5] aRay diagram correctly drawn showing that the ray passes through both surfaces undeflected, that is, the ray remains straight. [2] b angle of reflection r reflected ray incident and reflected rays correctly drawn [1] normal correctly drawn [1] angle of incidence correctly marked [1] angle of reflection correctly marked [1] 5 a 9 image mirror object 34 ray bends towards normal then away again so that it ends up parallel to original path [1] [1] [1] c Parallel rays remain parallel. [1] a converging [1] b closer than [1] c virtual; magnified [2] 10 a n = speed of light in a vacuum [2] speed of light in the material b n = refractive index, i = angle of incidence, r = angle of refraction [3] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 11 See Figure P13.05a in the Coursebook. 12 a 30° 30° A [6] 50° 50° Answers to end-of-chapter questions Chapter P14 Properties of waves 1 energy; matter [2] 2 a bounces off [1] b speed [1] B 3 reflection, refraction (in either order)[2] 4 a 4.0 cm [1] b 3.0 cm [1] c one wave = 4 cm so 10 cm = 2.5 waves [1] In block A, reflected ray at equal angle [1] and refracted ray bent away from normal. [1] so 2.5 waves pass in 1 s [1] In block B, reflected ray only, [1] frequency = 2.5 Hz [1] at equal angle. [1] 1 [1] there is only an internally reflected ray; [1] all of the ray is totally internally reflected. [1] 1 2 3 4 5 6 7 8 9 x / cm –2 parallel to axis to lens and on through focal point undeviated through centre of lens traced back to locate image 0 –1 13 a i any two of these three rays from top of object: as if from focal point to lens and then parallel to axis [2] 2 y / cm bWhen the angle of incidence is greater than the critical angle, d correct value of amplitude [1] correct value of wavelength [1] waves correctly reflected at barrier [1] separation remains as before [1] 5 [1] ii any two of: virtual / upright / magnified / further from lens / dimmer b i 3.4–3.6 cm [2] [1] ii magnifying glass[1] 14 a i image behind mirror image same distance from mirror, along line perpendicular to mirror [1] [1] ii reflected ray reaching eye [1] direction of reflected ray coming from image[1] b HIS, because S is not its own mirror image [1] c both rays straight on at first surface [1] 30° prism ray refracted down in air at second surface[1] 45° prism ray reflected down in glass at second surface[1] 90° reflection [1] straight on at third surface [1] 35 6 7 transverse describes a wave that varies from side to side, at right angles to the direction of travel longitudinal describes a wave that varies back and forth along the direction of travel Symbol Quantity v speed f frequency λ [1] Unit m/s [1] [1] Hz [1] wavelength [1] m [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 8 a decreases b stays the same c decreases [1] [1] [1] Answers to end-of-chapter questions b radio waves = lowest frequency, longest wavelength gamma rays = highest frequency, shortest wavelength[2] 9 a speed = frequency × wavelength, v = f λ[1] b v = f λ = 6 × 1014 × 3.75 × 10−7[1] = 2.25 × 108 m/s [1] 3 a false [1] b true [1] 10 4 300 000 000 m/s = 3.0 × 10 m/s [1] 5 a electromagnetic [1] short [1] c true [1] 8 b film or photographic film or electronic detector or charge-coupled device (CCD) [1] c absorbed/stopped by bone (not deflected/ reflected)[1] less absorption by flesh or penetrates/passes through flesh waves are curved in space beyond barrier [1] separation remains as before [1] 11 a i amplitude[1] ii wavelength b i string moves air [1] [1] backwards and forwards or up and down or produces compressions and rarefactions [1] ii gets quieter/softer/less loud [1] 12 a i R in correct position, by eye [1] iithree reflected waves correctly meeting mirror[1] three reflected waves equidistant and centred on R b first ray plus reflection correct second ray plus reflection correct reflected rays projected back, to meet behind mirror or labelled I and in correct position [1] [1] [1] [1] [2] b red = lowest frequency, longest wavelength violet = highest frequency, shortest wavelength [2] 2 a radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, gamma rays [2] 36 d any one of: photographic film badges, behind screen when operating X-ray machine, protective clothing, minimise exposure [1] Chapter P16 Magnetism 1 a i repel [1] ii attract [1] b See Figure P16.02 in the Coursebook. 2 a i [2] soft [1] ii hard [1] b i for example: steel [1] ii for example: soft iron [1] 3 a See Figure P16.04a in the Coursebook. [1] b See Figure P16.04b in the Coursebook. [1] 4 a one of the following: electromagnet can be switched on and off strength can be varied by changing current Chapter P15 Spectra 1 a red, orange, yellow, green, blue, indigo, violet [1] poles can be reversed by reversing current [1] b one of the following: N and S poles at opposite ends field lines have same pattern [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 5 a 2 repel 1 N Answers to end-of-chapter questions a left-hand end of solenoid N [1] right-hand end of solenoid S [1] attract S b lines of force out of N poles and into S poles 3 N N [1] lines close together at poles, farther apart elsewhere[1] S attract S N S repel each correct pair of attractive or repulsive forces [4] N repulsion indicated by distortion of pattern [1] b i S S N N S S with attractive forces shown [1] 6 a bigger current [1] more turns of wire or turns of wire closer together [1] add an iron core [1] b (for example) in a scrapyard crane or an electromagnetic door bolt [1] 7 a A soft magnetic material is easy to magnetise [1] and to demagnetise. [1] A hard magnetic material is difficult to magnetise and demagnetise. [1] b A hard material [1] because it retains its magnetisation well. c A soft material [1] [1] because its magnetisation can change easily. 8 [1] [1] Switch closed Switch open Soft iron magnetised loses its magnetism Steel magnetised keeps its magnetism attractive force [1] iii with soft iron core [1] iv can be switched on and off (or can be stronger)[1] 10 a can be switched off [1] can vary the strength [1] b i 1000 turns ii iron iii 3.0 A [2] Chapter P17 Electric charge 1 a rubbed, friction, opposite [3] b repel, attract [2] 2 a electrons [1] b negative [1] c positively [1] 3 Quantity Unit Symbol for unit force newton N electric charge coulomb C 4 a positive S N N S [2] ii N magnets in a square arranged N–S–N–S–N–S–N–S[1] 37 [1] 9 a (S) N S N 4 b similar pattern for both magnet and solenoid [4] [1] b They are equal. [1] c Suspend one so that it can turn freely. [1] Bring the other close to one end and observe repulsion.[1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 5 a i iron or ferromagnetic unmagnetised (before being brought near magnet)[1] (not non-magnetic) [1] Answers to end-of-chapter questions 5 cell ii magnet [1] b attracts (at first) [1] repels after touching or angle of thread increases as XY decreases [1] 6 a rub/rubbing [1] with dry cloth [1] b i current switch negative (−) [1] ii opposite charges attract [1] c horizontal arrow to L, starting or ending on sphere [1] d swings to R / moves away / is repelled [1] lamp a series circuit correctly drawn [1] correct symbols with labels [3] b at least two arrows around circuit [1] from positive of cell [1] c voltmeter [1] d volt (V) [1] + 6 – Chapter P18 Electrical quantities 1 a charge [1] b positive, negative [2] 2 a ammeter, series [1] b voltmeter, parallel A [1] 3 a R A V a correct symbols for resistor, ammeter and power supply[3] [4] V V b R = [1] I 4 38 Unit Potential difference volt [1] Current ampere Resistance ohm [1] Symbol for unit [1] V [1] A [1] Ω [1] connected in series [1] with voltmeter in parallel with resistor [1] b current [1] c potential difference (p.d.) [1] V d R = [1] I 6.5 [1] = 1.25 = 5.2 Ω [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 7 Equation In words In units Q = It charge = current × time [1] coulomb = ampere × second (C = A s) R= 8 Answers to end-of-chapter questions V I resistance = p.d. [1] current P = IV power = current × p.d. [1] E = IVt energy = current × p.d. × time [1] ohm = volt/ampere (Ω = V/A) [1] watt = ampere × volt (W = A V) [1] joule = ampere × volt × second (J = A V s) Q a I= [1] t 30 = [1] 20 = 1.5 A [1] 9 = 1.5 × 10 × 20 [1] = 300 J [1] a i [1] 10 a light [1] b heat [1] c power = 36 W [1] d energy = power × time [1] = 36 × 60 [1] b E = Pt[1] = IVt[1] [1] = 2160 J [1] P e I = [1] V 30 [1] = 12 = 3 A [1] A Chapter P19 Electric circuits coil of wire 1 a current [1] b sum [1] each symbol correctly drawn [4] 2 V battery/cell, ammeter, coil in series [1] voltmeter in parallel with coil [1] standard symbols used for battery/cell, voltmeter and ammeter [1] V ii R = [1] I iii any two of: length (of wire) diameter/cross-section/ area (of wire) resistivity/type of material temperature[2] 6.0 b R = [1] 1.5 = 4.0 Ω [1] 39 resistance of AB = 1.0 Ω [1] resistance per metre = 0.50 Ω/m [1] 3 a melting, burning, fumes [1] b wire melts, breaks circuit [1] c Fuse will not break for normal current, but will break when current rises above this value. [1] 4 a voltage (or p.d.) [1] b shared [1] c more (greater) [1] 5 a series [1] b parallel [1] c series [1] d parallel [1] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 6 Name of device 9 Description light-dependent resistor (LDR) [1] resistance decreases [1] when light falls on it thermistor [1] resistance [1] changes when temperature changes 7 a 8 Circuit symbol 6V series circuit [1] correct symbols for resistor, switch and power supply [3] b 10 + 40 [1] = 50 Ω [1] c 0.12 A [1] d 0.12 A [1] a wires overheat (risk of fire) [1] b fuse, trip switch [2] c Use thicker wires, [1] which have lower resistance, [1] so there is less heating. [1] a in parallel [1] b 6.0 V [1] across each resistor c The 2 Ω resistor, because the resistance is lower. [1] Answers to end-of-chapter questions 1 1 1 ii = + [1] R 3 6 R = 2 Ω [1] V [1] c I= R = 6.0 A [1] d i stays the same [1] ii decreases [1] Chapter P20 Electromagnetic forces 1 current, magnetic, circles (or circular), wire [4] 2 current, magnetic, turning, rotate [4] 3 a The wire will swing the other way. [1] b The wire will swing the other way. [1] 4 a force (motion) [1] b magnetic field [1] c current [1] 5 a downwards [1] b to the right, [1] by Fleming’s left-hand rule [1] 6 a downwards [1] b upwards [1] c The forces are unbalanced, [1] and so provide a turning effect. d The force is zero, [1] because the current does not cut across the magnetic field (it is parallel to the field). 7 a i [1] [1] current clockwise when viewed from top [1] ii anticlockwise or down on left and/or up on right [1] b i [1] faster or greater turning effect [1] ii faster or greater turning effect [1] [1] iii faster or greater turning effect [1] 1 1 1 d = + [1] R 2 3 3 2 5 + = [1] 6 6 6 6 [1] R = = 1.2 Ω 5 V 6 R = = [1] R 1.2 = 5.0 A[1] Chapter P21 Electromagnetic induction 1 conductor, magnetic, induced, circuit, current [5] 2 (answers from the top) d.c.; a.c.; a.c.; d.c.; a.c.; d.c.; a.c. [7] 3 coil, rotate/turn, magnetic, e.m.f., current [5] 4 high, smaller, less [3] 5 a primary, core, secondary [3] 10 a i 4.0 V [1] b step-up, e.m.f./voltage [2] ii 12 V [1] c step-down, e.m.f./voltage [2] b i 6 Ω [1] 40 © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences Answers to end-of-chapter questions 6 Vp = p.d. across primary coil d stronger magnetic field [1] turn the coil faster [1] Vs = p.d. across secondary coil Np = number of turns on primary coil Ns = number of turns on secondary coil [4] 11 a 7 Ip = current in primary coil Vp = p.d. across primary coil Vp Np = [1] Vs Ns 5000 × 12 [1] Ns = 230 = 261 [1] b Ip × Vp = Is × Vs[1] Is = current in secondary coil Vs = p.d. across secondary coil [4] 8 aThe magnetic field around the wire is changing (it is cutting across field lines). 0.40 × 12 [1] 230 = 0.021 A [1] Ip = [1] bIt will change sign / direction (from positive to negative, or the other way round). [1] c She should move the wire more quickly. [1] d No, [1] Chapter P22 Atomic physics 1 electron nucleus proton because it is not cutting across the field lines / the magnetic field is not changing. [1] 9 a so that less energy is lost during transmission [1] Vp Np = [1] Vs Ns Vs = Vp × Ns[1] Ns 3 × 200 = 60 V [1] 10 cUse the primary coil as the secondary and the secondary as the primary. [1] b 10 a i X: coil [1] ii Y: slip rings [1] iii Z: brushes [1] b A.c. flows back and forth, changing direction. D.c. flows in one direction only. [1] d.c. Current neutron 4 2 He 2 Name What it tells us X chemical symbol [1] name of element [1] Z proton number [1] number of protons in nucleus [1] A nucleon number [1] number of nucleons in nucleus [1] 3 proton number + neutron number = nucleon number [1] 4 a different numbers [1] b the same number [1] c different numbers [1] 5 a 6 protons [1] Time b 6 neutrons [1] a.c. c 6 electrons [1] 6 a 79 + 118 – = 197 197 79 [1] [1] [1] b c more turns [1] 7 a 19 [1] bigger area [1] b 39 [1] correct (labelled) diagram c 41 Symbol [1] + 0 [4] 40 19 Au[2] K[2] © Cambridge University Press 2017 Cambridge IGCSE Combined and Co-ordinated Sciences 8 Radiation alpha Penetration least penetrating gamma in between most penetrating [1] Absorption most easily in between least easily absorbed [1] absorbed [1] Absorbed by thin paper, a few cm of air 7 9 beta thin metal thick lead or foil [1] concrete [1] a (average) time, half, decay [3] b See Figure P22.08 in the Coursebook.[3] 10 β is more penetrating than α. [1] Detect using Geiger counter. [1] Place thin paper over sources − α does not pass through.[1] Place thin aluminium foil over sources − neither passes through. [1] 11 a it has negative charge [1] 12 42 b charged; Fleming’s left-hand rule [2] c it is uncharged [1] Use … because … Finding the age of an object radioactive substances decay at a known rate. Seeing through solid objects radiation can penetrate matter. Sterilising medical equipment radiation can destroy living cells. Tracing the movement of hazardous substances small amounts of radiation can be detected. Answers to end-of-chapter questions 13 a i 3 [1] ii 3 [1] iii 4 [1] iv 3 + 4 = 7 [1] b 14 a Li [1] 7 3 Particle Charge Mass electron −1 m neutron 0 [1] 2000m [1] proton +1 [1] 2000m [1] b i 92 [1] ii 146 [1] iii 92 [1] 15 a i proton [1] ii proton and neutron [1] b number of protons = 47 [1] number of neutrons = 107 − 47 = 60 c i 8 h ± 0.25 h [1] [1] iiChoose two points on the graph; for each, halve the value and add 8 h to the time. [2] [4] © Cambridge University Press 2017


