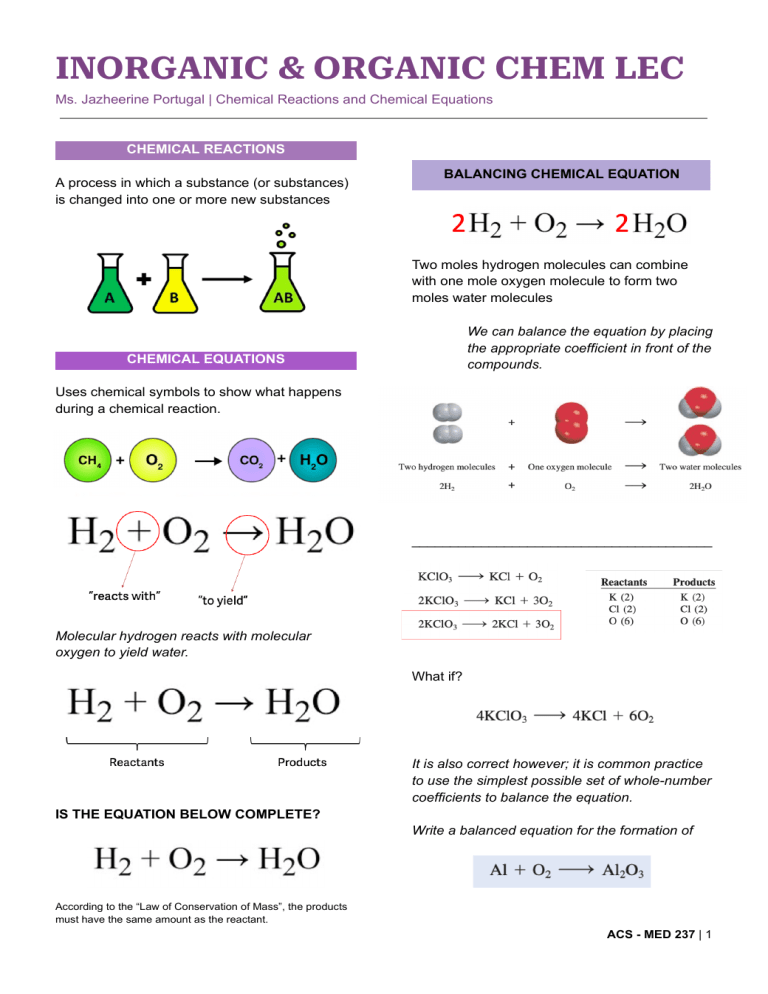
INORGANIC & ORGANIC CHEM LEC Ms. Jazheerine Portugal | Chemical Reactions and Chemical Equations CHEMICAL REACTIONS A process in which a substance (or substances) is changed into one or more new substances BALANCING CHEMICAL EQUATION Two moles hydrogen molecules can combine with one mole oxygen molecule to form two moles water molecules We can balance the equation by placing the appropriate coefficient in front of the compounds. CHEMICAL EQUATIONS Uses chemical symbols to show what happens during a chemical reaction. _______________________________________ Molecular hydrogen reacts with molecular oxygen to yield water. What if? It is also correct however; it is common practice to use the simplest possible set of whole-number coefficients to balance the equation. IS THE EQUATION BELOW COMPLETE? Write a balanced equation for the formation of According to the “Law of Conservation of Mass”, the products must have the same amount as the reactant. ACS - MED 237 | 1 BALANCING CHEMICAL EQUATION TYPES OF CHEMICAL REACTIONS To provide additional information, chemists indicate physical states of reactants and products by using the letters g, l and s to denote gas, liquid and solid. INTERPRETATION OF CHEMICAL EQUATION Chemical equations are used to denote the type of chemical reaction involved and the quantity of reactants and products present (Stoichiometry). Chemical equations are used to denote the type of chemical reaction involved and the quantity of reactants and products present (Stoichiometry). Chemical equations are used to denote the type of chemical reaction involved and the quantity of reactants and products present (Stoichiometry). Chemical equations are used to denote the type of chemical reaction involved and the quantity of reactants and products present (Stoichiometry). 2 STOICHIOMETRY To interpret a reaction quantitatively, we need to apply our knowledge of molar masses and the mole concept. Stoichiometry is the quantitative study of reactants and products in a chemical reaction. The stoichiometric coefficients in a chemical equation can be interpreted as the number of moles of each substance. 3 Or EXAMPLE: 16.0 g of H2 react completely with N2 to form NH3. How many grams of NH3 will be formed? CHALLENGE : The food we eat is degraded or broken down in our bodies to provide energy for growth and function. A general overall equation for this very complex process represents the degradation of Glucose (C6H12O6) to carbon dioxide (CO2) and water (H2O) : (C6H12O6) + 6O2 —> 6CO2 + 6H2O If 856 g of (C6H12O6) is consumed by a person over a certain food, what is the mass of CO2 produced? 4 CHALLENGE: All Alkali metals react with water to produce hydrogen gas and the corresponding alkali metal hydroxide. A typical reaction is between lithium and water. 2Li (s) + 2H2O (l) —> 2 LiOG (aq) + H2 (g) How many grams of Li are needed to produce 9.89 of H2? 5