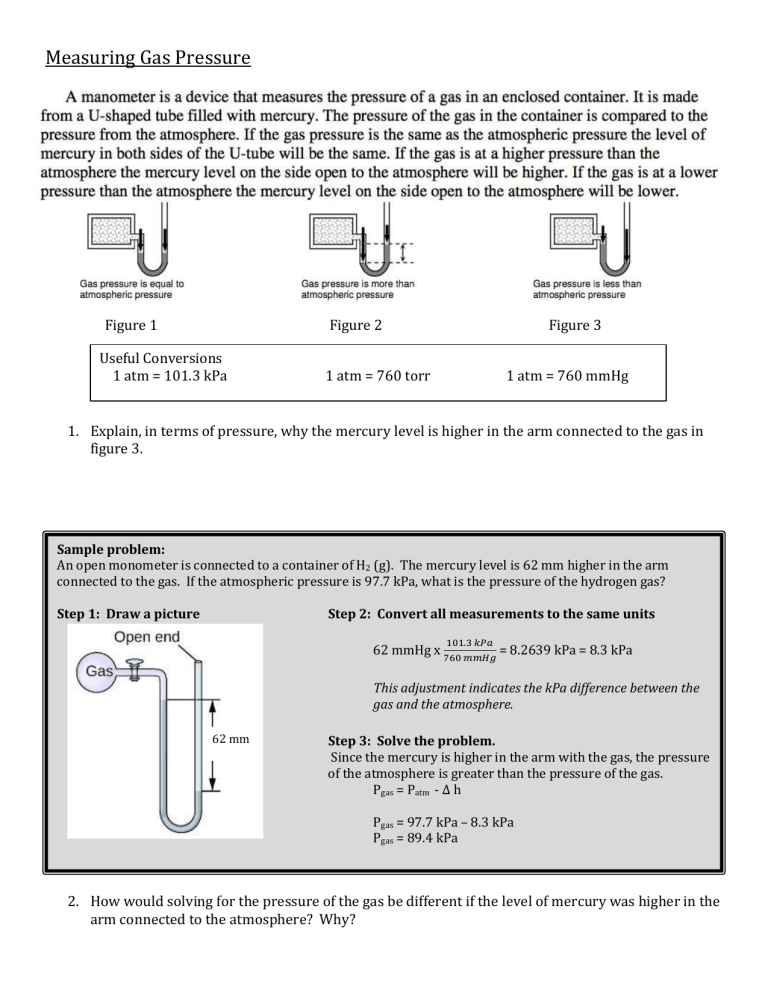
Measuring Gas Pressure Figure 1 Figure 2 Useful Conversions 1 atm = 101.3 kPa Figure 3 1 atm = 760 torr 1 atm = 760 mmHg 1. Explain, in terms of pressure, why the mercury level is higher in the arm connected to the gas in figure 3. Sample problem: An open monometer is connected to a container of H2 (g). The mercury level is 62 mm higher in the arm connected to the gas. If the atmospheric pressure is 97.7 kPa, what is the pressure of the hydrogen gas? Step 1: Draw a picture Step 2: Convert all measurements to the same units 101.3 𝑘𝑃𝑎 62 mmHg x 760 𝑚𝑚𝐻𝑔 = 8.2639 kPa = 8.3 kPa This adjustment indicates the kPa difference between the gas and the atmosphere. 62 mm Step 3: Solve the problem. Since the mercury is higher in the arm with the gas, the pressure of the atmosphere is greater than the pressure of the gas. Pgas = Patm - ∆ h Pgas = 97.7 kPa – 8.3 kPa Pgas = 89.4 kPa 2. How would solving for the pressure of the gas be different if the level of mercury was higher in the arm connected to the atmosphere? Why? Practice Problems 3. An open manometer, as pictured to the right is used to measure the pressure of neon gas. The mercury is 510 mm higher in the arm connected to the gas. Determine the pressure exerted by the gas, if the atmospheric pressure is 126.6 kPa. 126.6 kPa X kPa 510 mm Hg 4. An open manometer, is filled with mercury and connected to a container of nitrogen. The mercury level is 623 mm higher in the arm of the tube connected to the air, which has a pressure of 115.4 kPa. What is the pressure of the nitrogen gas in kPa? 115.4 kPa 623 mm Hg 5. A container of helium is connected to a manometer and the mercury level is 145 mm lower on the side open to the atmosphere. Determine the pressure of the helium (in kPa) if the atmospheric pressure is 775 mmHg. 6. A sample of carbon dioxide is contained and connected to a manometer. The mercury level is 71 mm higher in the arm connected to the atmosphere. If the atmospheric pressure is 1.05 atm determine the pressure, in kilopascals, of the carbon dioxide gas. Measuring Gas Pressure Directions: Answer each of the following questions using your knowledge of chemistry and the diagrams to the right. 1. Explain, in terms of the Kinetic Molecular theory why the level of mercury moves in the arm of the manometer when the value to the gas is opened. 2. Which figure contains a gas that exerts a greater pressure than the atmosphere? How do you know? 3. Calculate the pressure exerted by the gas in figure (ii) given that the atmospheric pressure is 101.3 kPa. Measuring Gas Pressure Directions: Answer each of the following questions using your knowledge of chemistry and the diagrams to the right. 1. Explain, in terms of the Kinetic Molecular theory why the level of mercury moves in the arm of the manometer when the value to the gas is opened. 2. Which figure contains a gas that exerts a greater pressure than the atmosphere? How do you know? 3. Calculate the pressure exerted by the gas in figure (ii) given that the atmospheric pressure is 101.3 kPa.