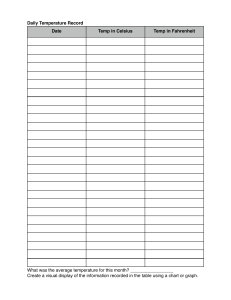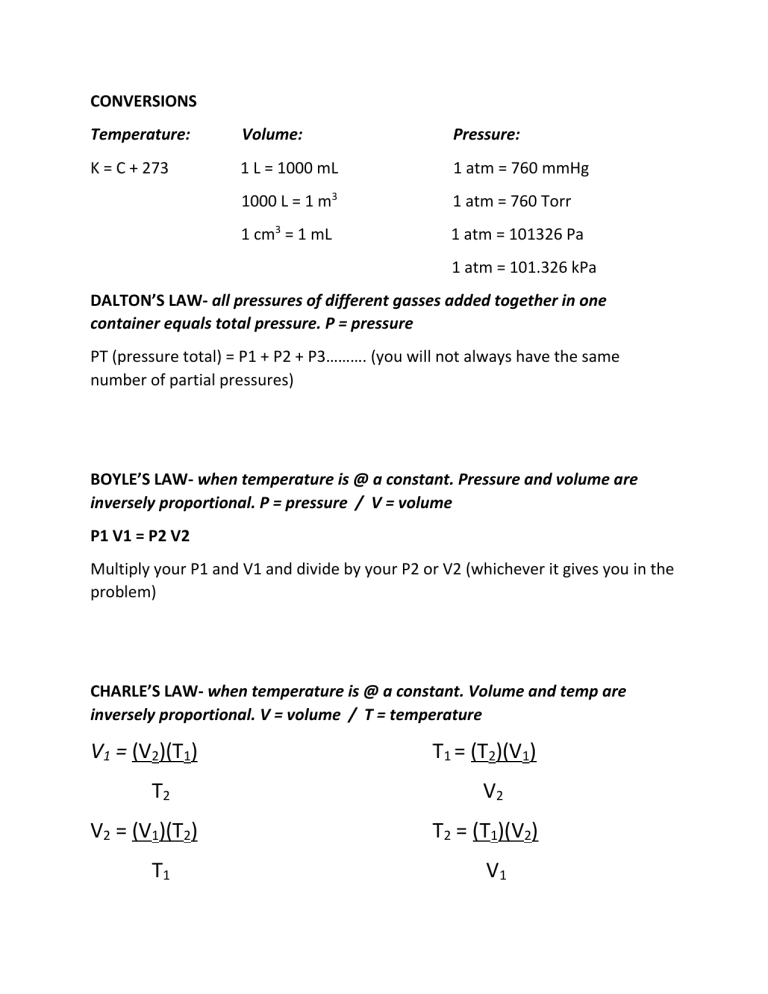
CONVERSIONS Temperature: Volume: Pressure: K = C + 273 1 L = 1000 mL 1 atm = 760 mmHg 1000 L = 1 m3 1 atm = 760 Torr 1 cm3 = 1 mL 1 atm = 101326 Pa 1 atm = 101.326 kPa DALTON’S LAW- all pressures of different gasses added together in one container equals total pressure. P = pressure PT (pressure total) = P1 + P2 + P3………. (you will not always have the same number of partial pressures) BOYLE’S LAW- when temperature is @ a constant. Pressure and volume are inversely proportional. P = pressure / V = volume P1 V1 = P2 V2 Multiply your P1 and V1 and divide by your P2 or V2 (whichever it gives you in the problem) CHARLE’S LAW- when temperature is @ a constant. Volume and temp are inversely proportional. V = volume / T = temperature V1 = (V2)(T1) T2 V2 = (V1)(T2) T1 T1 = (T2)(V1) V2 T2 = (T1)(V2) V1 AVAGADRO’S LAW- if temp and pressure are constant, volume and # of moles are directly proportional. V = volume / N = # of moles V1 = (V2)(N1) N2 V2 = (V1)(N2) N1 N1 = (V1)(N2) V2 N2 = (N1)(V2) V1 IDEAL GAS LAW- the product of P and V of 1 gram of a molecule will equal the product of the absolute temp. and the universal gas constant. P = pressure / V = volume / N = # of moles / R = match to the pressure unit / T = temp in kelvin P = (N)(Rt) V V = (N)(Rt) P N = Pv Rt T = Pv nR