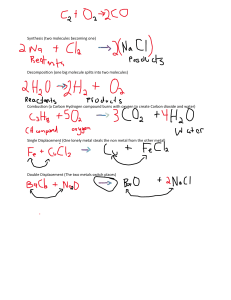
METALS METALS OBJECTIVES • To familiarize with the different properties of metals. • To understand the forming processes and performance of metals. • To differentiate Ferrous from Non – Ferrous metals. • To know the environmental impact of metals and how it is disposed. • To appreciate the new innovation involving the use of metals. ALL ABOUT METALS Metals are a crucial element of our existence, even if we don’t realize it. It is very strong and durable therefore is used to make many things. The periodic table of elements consist of 118 elements, of which 92 are metals. IRON COBALT NICKEL COPPER SILVER GOLD ZINC CADMIUM MERCURY STRUCTURE OF METAL FACE CENTERED CUBE, PF = 74% BODY CENTERED CUBE, PF = 68% HEXAGONAL CLOSE – PACKED, PF = 74% PROPERTIES OF METALS Prepared by: Samson, Christian Angelo VALENCY Metals typically have 1 to 3 electrons in the outermost shell of their atoms CONDUCTION Metals are good conductors because they have free electrons. Silver and copper are the two best conductors of heat and electricity. Lead is the poorest conductor of heat. Bismuth, mercury and iron are also poor conductors LUSTER Metals have the quality of reflecting light from their surface and can be polished e.g., gold, silver and copper. MALLEABILITY Metals have the ability to withstand hammering and can be made into thin sheets known as foils. DUCTILITY Metals can be drawn into wires. HARDNESS All metals are hard except sodium and potassium, which are soft and can be cut with a knife. DENSITY Metals have high density and are very heavy. Iridium and osmium have the highest densities whereas lithium has the lowest density MELTING AND BOILING POINT Metals have high melting and boiling points. Tungsten has the highest melting and boiling points whereas mercury has the lowest. Sodium and potassium also have low melting points. STATE Metals are solids at room temperature with the exception of mercury, which is liquid at room temperature (Gallium is liquid on hot days). MANUFACTURING PROCESS AND PERFORMANCE OF METALS Prepared by: Peralta, Gem Liu L. MERRY JOY TRUPA • • Finishes 2nd in the Standard Distance Duathlon in the 2023 PTO Asian Open in Singapore Reigning Powerman Malaysia Champion METALS Metal Manufacturing Processes Casting Deformation Material Removal Casting Processes Involve pouring molten metal into a mold cavity where, once solid, the metal take on the shape of the cavity. • • • Expendable mold casting Permanent mold casting Power metallurgy Expendable Mold Casting Where the mold must be destroyed in order to remove the part. Permanent Mold Casting • For which the mold is fabricated out of a ductile material and can be used repeatedly. Powder metallurgy In powder metallurgy a metal powder is compacted into the desired shape and heated to cause the particles to bond into a rigid mass. Metal Manufacturing Processes Casting Deformation Deformation Processes These processes use plastic deformation resulting from the use of a tool that applies stresses to the piece which exceed the yield stress of the metal. • • Bulk Processes Sheet Metalworking BULK PROCESSES It is characterized by large deformations and shape changes and by the fact that the surface area to volume ratio is relatively small. SHEET METALWORKING • Is a manufacturing process of cutting and forming relatively thin metal sheets, strips, and coils to create desired shape sheet metal parts. Forging Bending Deep Drawing Rolling Extrusion Wire Drawing Shearing Metal Manufacturing Processes Casting Deformation Material Removal MATERIAL REMOVAL PROCESSES • These processes remove extra material from the workpiece in order to achieve the desired shape. • • • Machining Operations Abrasive Machining Nontraditional Processes MACHINING OPERATION • These are cutting operations using cutting tools that are harder than the metal of the product. ABRASIVE MACHINING • In these methods material is removed by abrasive particles that normally form a bonded wheel. NONTRADITIONAL PROCESSES • These methods use lasers, electron beams, chemical erosion, electric discharge and electrochemical energy instead of traditional cutting and grinding tools. PERFORMANCE OF METALS HARDNESS TESTING TENSILE TESTING HARDNESS TESTING ❑ Uses a carbide ball indenter to measure hardness on course or uneven materials Brinell ❑ Uses a light load to test hardness indentations in a range of materials Vickers & Knoop ❑ Measures the depth of penetration on soft or hard metal materials Rockwell HARDNESS TESTING TENSILE TEST TENSILE TEST METAL ALLOYS FERROUS NON- FERROUS FERROUS METALS PROPERTIES AND APPLICATIONS YT: UNDERSTANDING METALS FERROUS METALS ARE ALLOYS PRIMARILY COMPOSED OF IRON WITH VARYING AMOUNTS OF CARBON AND OTHER ELEMENTS. • They are widely used in industries such as construction, automotive, aerospace, and manufacturing due to their desirable properties. YT: PRODUCTION OF ALLOYS FERROUS STEEL CAST IRON STEEL PLAIN CARBON STEEL LOW CARBON STEEL MEDIUM CARBON STEEL ALLOY STEEL HIGH CARBON STEEL TOOL STEEL STAINLESS STEEL A. PLAIN CARBON STEEL • Iron and carbon A. LOW CARBON STEEL • Contains less than about 0.25 wt.%C (Mild steel) • High tensile strength, Ductile, and quite tough. • Poor resistance to corrosion. • Used for wires, bolts and pipes. A. PLAIN CARBON STEEL B. MEDIUM CARBON STEEL • Contains about 0.25 -0.6 wt.%C • Higher strength and lower ductility - less moldable under pressure. • Good wear resistance. • Used for gears and railroad tracks. A. PLAIN CARBON STEEL C. HIGH CARBON STEEL • • • • Contains about 0.6 -1.4 wt.%C Hardest, toughest, and least ductile carbon steels. Difficult to weld Applications: hammer, knives, saw blades, springs, gear wheels B. ALLOY STEEL: • Made of iron, carbon, and other elements such as silicon, nickel, chromium, and etc. TOOL STEEL • High-speed steel - Withstand higher temperatures without losing its hardness and toughness. • Used in reamers, drill bits, and rotating cutting tools. B. ALLOY STEEL: STAINLESS STEEL • High resistance to corrosion in a variety of environment. • Pre-dominant alloy: Chromium (at least 11wt.%) • Ex. 18/8 stainless steel -18% chromium and 8% nickel. CAST IRON GREY CAST IRON MALLEABLE CAST IRON WHITE CAST IRON GRAY CAST IRON: • • • • • Carbon contents vary from 2.5-4.0wt.%, 1.0-3.0% Si, and 0.4-1.0% Mn. The graphite flakes in gray iron allow for better lubrication. Least expensive and most common type Good Bearing surface, Vibration damping, machinability Molded state poured into a sand mold to be cooled slowed slowly obtaining grey cast iron. Application: • Gears, Pumps, Linkages, Stove parts, Steering knuckles, camshafts and engine blocks. GRAY CAST IRON: • • • • • Carbon contents vary from 2.5-4.0wt.%, 1.0-3.0% Si, and 0.4-1.0% Mn. The graphite flakes in gray iron allow for better lubrication. Least expensive and most common type Good bearing surface, Vibration damping, machinability Sand mold to be cooled slowly obtaining grey cast iron. Application: • Linkages, Stove parts, Steering knuckles, camshafts, and engine blocks. WHITE CAST IRON • • • • Carbon 1.8 % -3.6 %, Silicon 0.5 % -1.9 %, and manganese (Mn) 1 % – 2 %. Hardest of all Cast Irons and is wear-resistant. It has high tensile strength but weak compressive strength. It is not easily machinable due to its hardness. Application: • Used for car wheels, rollers for crushing grains, crusher jaw plates, etc. MALLEABLE CAST IRON • • • • It is a white cast iron that has been annealed. Highly shock resistant. Malleable iron is better suited for decorative elements Used for small castings requiring good tensile strength and the ability to flex without breaking (ductility). Application: • Connecting rods, transmission gears, and pipe fittings and valve parts. NON-FERROUS METAL ❑ Non-ferrous metals are alloys or metals that do not contain any appreciable amounts of iron. ❑ All pure metal are non-ferrous metals CHARACTERISTICS OF NON FERROUS METAL ✓ Easy to fabricate (including machinability, casting, and welding) ✓ High corrosion resistance ✓ Good thermal and electrical conductivity ✓ Low density ✓ Non-magnetic ✓ Colorful PROCESS FOR NONFERROUS METAL ➢Non-ferrous metals are usually obtained from minerals like carbonates, silicates and sulphones before being refined through electrolysis. Ferrous Vs NonFerrous USES AND PROPERTIES OF NON-FERROUS METAL ➢COPPER ❖The properties of copper and its alloys include high thermal conductivity, high electrical conductivity, good corrosion resistance, and high ductility. USES AND PROPERTIES OF NON-FERROUS METAL ➢ALUMINUM ❖Aluminum is an important metal that is used in a wide range of applications due to its low weight and ease of machining USES AND PROPERTIES OF NON-FERROUS METAL ➢LEAD ❖Lead is the heaviest common metal and is resistant to corrosion. It also doesn’t react with many chemicals and is soft and malleable USES AND PROPERTIES OF NON-FERROUS METAL ➢ZINC ❖Zinc has been used for centuries as an alloying element, particularly to alloy steel for a range of purposes as well as alloying copper to create brass USES AND PROPERTIES OF NON-FERROUS METAL ➢SILVER ❖Silver has been used as a precious metal for centuries. With the highest electrical conductivity, thermal conductivity and reflectivity of any metal, silver is also soft and malleable when heated and is highly resistant to corrosion USES AND PROPERTIES OF NON-FERROUS METAL ➢GOLD ❖Gold is the most malleable of metals as well as being ductile and resistant to corrosion and many other chemical reactions USES AND PROPERTIES OF NON-FERROUS ALLOYS ➢BRASS and BRONZE ❖These alloys melt at lower temperatures than ferrous materials and cast well, making them ideal for decorative applications. ❖ Bronze and brass are corrosion resistant, even in the presence of salt, and so are widely used. ❖Brass is created as an alloy of copper and zinc, while bronze is an alloy of copper with aluminums and/or nickel Environmental impact of metals Prepared by: Zyrene Shane L. Cordero Heavy metals are well-known environmental pollutants owing to their toxicity, longevity in the atmosphere, and ability to accumulate in the human body via bioaccumulation. The pollution of terrestrial and aquatic ecosystems with toxic heavy metals is a major environmental concern that has consequences for public health. Heavy metal pollution has emerged due to anthropogenic activity which is the prime cause of pollution, primarily due to mining the metal, smelting, foundries, and other industries that are metal-based, leaching of metals from different sources such as landfills, waste dumps, excretion, livestock and chicken manure, runoffs, automobiles and roadworks. SOURCES OF HEAVY METALS POLLUTION Natural Sources: This includes natural events such as volcanic eruption and weathering of rocks/soil. Anthropogenic Sources: This includes human activities such as pharmacy, industrial, agricultural, and mining activities. Domestic effluents are also a significant source of heavy metal pollution. EFFECTS OF HEAVY METAL POLLUTION ON THE ENVIRONMENT 1. Water contamination: Heavy metal pollution causes water contamination/water pollution. This lead to degradation of water quality/loss of water quality and water is not suitable for drinking purpose. 2. Groundwater Pollution: Due to heavy rainfall the heavy metals present in the solid waste can join the leachate and may reach groundwater. The contamination of groundwater with heavy metals poses negative threats to the ecosystem. 3. Soil Contamination: Heavy metals contaminate soil leading to soil pollution. Urban runoff or agriculture runoff containing heavy metals can contaminate the surface water. 4. Air pollution: Heavy metals are contaminating the air due to fossil fuel combustion, industrial activities, etc. Air contaminated with heavy metals has negative impacts on the lungs. 5. Marine Ecosystem: Heavy metal pollution adversely impacts the marine ecosystem. Bioaccumulation and biomagnifications is one of the environmental issues associated with heavy metal pollution. Heavy metal pollution can have detrimental health effects on marine animals. Some fish species are sensitive to heavy metals and cannot survive in water contaminated with heavy metals. 6. Vegetation Contamination: Heavy metals also negatively impact plant growth cause plant diseases and death of plants due to bioaccumulation. A few of the most frequent heavy metals that contaminate the environment include mercury, cadmium, arsenic, chromium, nickel, copper, and lead. ▪ Cadmium pollution of the aquatic environment occurs through absorption, industrial waste, and surface runoff into sediments soil and sediments. People can be poisoned by cadmium via ingesting food, breathing air, or drinking water rich in the metal. Cadmium does not have any attributes that are helpful for plant growth and metabolic processes 78 ▪ Mercury is an extremely hazardous heavy metal that may be found in biosphere. Mercury converts to the highly toxic methylmercury when in contact with aquatic sediments (Gworek et al., 2020). Methylmercury enters the human body through the food chain via fish, seafood, and wildlife, which become contaminated after ingestion of toxic microorganisms. It penetrates the circulation after being absorbed into the human body and causes a variety of neurological problems (Rice et al., 2014). ▪ Lead is toxic to the human body when exposed to amounts greater than the optimum. It is toxic to the nervous system, heart, kidneys, and gastrointestinal tract, with the neurological system being the most vulnerable. Lead also causes cognitive issues in children by interfering with brain growth. 79 ▪ ▪ ▪ Manganese, the most plentiful of the toxic heavy metals, is found in various oxidation states in nature. During combustion of methylcyclopentadienyl manganese tricarbonyl (MMT), an additive in gasoline, manganese oxides are emitted into the air. Chromium is a cancerous and toxic element. In the environment, it exists in two stable oxidation states: chromium (III) and chromium (IV) (VI). Chromium (III) is a less hazardous form of chromium (VI). Long-term exposure can affect the liver, kidneys, circulatory system, and nerves, as well as cause skin irritation. High levels of breathing can cause nasal irritation, nose ulcers, runny nose, and breathing problems such as asthma, cough, shortness of breath, or wheezing Cobalt is found in abundance across the environment, such as vegetation, soils, rocks, and water and is utilized to make alloys. Although its rate of discharge is low, it is highly dangerous to humans. 80 ▪ Nickel is a naturally abundant element and has extensive industrial uses. It has many adverse effects on humans, and causes allergies, nasal and lung cancer, and kidney and cardiovascular diseases owing to the inhalation of contaminated air (Genchi et al., 2020, Lu et al., 2005). ▪ Copper deficiency alters important metabolic processes, and elevated exposure causes toxicity. Excessive copper use can cause major health problems including high cholesterol, rapid breathing, kidney and liver damage, convulsions, cramps, vomiting, and even death ▪ Zinc toxicity depends on the manner and quantity of exposure. Too much zinc can cause serious health problems such as stomach pains, rashes, vomiting, nausea, and anemia. Vomiting, diarrhea, bloody urine, icterus (yellow mucus membrane), liver failure, renal failure, and anemia have all been documented as symptoms of zinc toxicosis. 81 ▪ Antimony is a poisonous element that may be found in nanogram amounts in the air. Natural occurrences, including volcanic activity and weathering, as well as anthropogenic activities, cause emissions into the atmosphere (He et al., 2019a). Antimony toxicity develops in those working in industrial areas from inhalation (Fig. 1). Antimony poisoning causes physiological shortcomings, including pancreatitis, cardiotoxicity, and respiratory problems (pleural adhesions, chronic emphysema, chronic bronchitis, respiratory irritation, and inactive tuberculosis). It is also carcinogenic and affects reproduction (Sundar and Chakravarty, 2010). 82 ▪ Thallium is found in the environment in many forms and is hazardous to biological organisms. Thallium's toxicity is greater than any other heavy metal. Exposure to thallium is extremely harmful to humans. 83 Metal Recycling Metals are essential, versatile and can be used in a number of ways. Metals can be used for industrial purposes such as the manufacture of trucks, cars, airplanes, ships, and railways. They can also be used to manufacture domestic items such as cutlery, crockery and even in packaging. The good thing about metal recycling is that metal can be recycled over and over without altering its properties. Metal Recycling process Collection This is the first and most important step in metal recycling. It simply involves collecting all materials that are made of metals. This process should be organized in such a way that there should be containers specifically designed to collect metals. Sorting Once the metals have been collected, the next important step is to sort the metals. This involves separating what can be recycled form what is nonrecyclable. Processing All the recycled materials are squeezed and squashed using machines so that they do not occupy so much space in the conveyor belts. Shredding The metals are broken down into tiny pieces or sheets to allow further processing. The small pieces have a large surface to volume ratio that can be melted using less energy as compared to when they are in large pieces of metal. Normally, steel is changed into steel blocks while, on the other hand, aluminum is converted into sheets. Metal Recycling process Melting Melting of the scrap metal takes place in a large furnace. Each metal is taken to a furnace that is specifically designed to melt that particular metal based on its specific properties. Purification Metals are purified using different methods. Purification of metals is done to ensure that the final product is free of impurities and that it is of high quality. Electrolysis is one of the methods of purifying metals. Solidifying of the Metal The molten metal is then carried by the conveyor belt to a cooling chamber where it is cooled and solidified. It is at this stage that the scrap metal is made into a solid metal that can be used again. Other chemicals are then added into the molten metal to make it acquire its density and other properties. references https://www.sciencedirect.com/science/article/pii/S1018364722000465#:~:text=Most%20heavy %20metals%20cause%20environmental,them%20through%20the%20food%20chain . https://www.sciencedirect.com/science/article/pii/S2405844020315346#sec8 https://www.intechopen.com/chapters/81083 https://www.envpk.com/impact-of-heavy-metal-pollution-on-the-environment/ https://www.conserve-energy-future.com/recyclingmetal.php NEW INNOVATION INVOLVING METALS PREPARED BY: ESTABILLIO, JOYCE B. We are inspired by innovations to broaden our perspectives and look into new industrial and technical possibilities. All currently operating industrial and scientific fields can be improved by fusing cutting-edge technology with expertise. HIGH STRENGTH STEEL For instance, they are crucial in the automotive industry, where producers aim to create lighter, cleaner, and more cost-effective vehicles. Due to its unique properties, maraging steel was used extensively in the military to create rocket engine hulls, aircraft catapults, and weapon barrels. HYDROGEN METALLURGY Hydrogen metallurgy is a technique that uses hydrogen instead of carbon as a reduction agent to minimize CO2 emissions, and is advantageous to the steel industry's long-term sustainability. Direct reduction of iron (DRI) DRI is created by removing oxygen from iron ore in its solid form. This technique covers a wide range of processes that use diverse reactors and reducing agents. DRI operations can minimize CO2 emissions by utilizing natural gas instead of coal. Automated processes and digitalization Digitalization will also make it easier to check surfaces and working parts in realtime to determine the quality of the completed product and to systematize faults. The strategy of the metallurgy industry will continue to develop in the following directions: • meeting future demands on new products encouraging product innovation, for new social and economic problems solutions; • constant improvement of materials’ characteristics and performance; • optimization of exploration, manufacturing, processing, and recycling; • empowering modern technologies and infrastructure; These changes will have a significant impact on both the global economy and society's perception of itself.