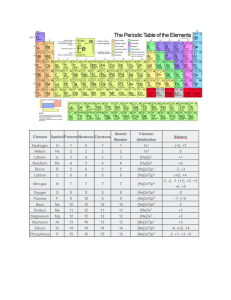
How Elements Heavier Than Iron Were Formed LIMITATIONS OF BIG BANG/STELLAR NUCLEOSYNTHESIS Fusion reactions above Fe is unfavorable • Nuclear binding energy per nucleon holds the nucleus intact • Smaller nuclear binding energy per nucleon • Further fusion reactions with Fe require more energy LIMITATIONS OF BIG BANG/STELLAR NUCLEOSYNTHESIS Nucleosynthesis of elements beyond Fe are nonspontaneous and require different pathways • Neutrinos released by a supernova help in forming neutrons and protons which then get captured by nuclei residing in nearby stars • Neutron or proton capture processes help in achieving higher-level nucleosynthesis NEUTRON CAPTURE Neutron capture starts with a neutron being added to a seed nucleus This starting reaction would then produce a heavier isotope of the element NEUTRON CAPTURE Beta decay results in an increase in the number of protons of the nucleus by one A heavier nucleus of a new element is formed NEUTRON CAPTURE: S-PROCESS Slow neutron capture or s-process happens when there is a small number of available neutrons • The rate of neutron capture is slow compared to the rate of beta decay (hence the term slow) • If a beta decay occurs, it almost always occurs before another neutron can be captured NEUTRON CAPTURE: S-PROCESS Slow neutron capture or s-process happens when there is a small number of available neutrons • These occur mainly on red giant or supergiant stars, with each neutron capture taking a decade and the cascade of processes taking thousands of years to complete NEUTRON CAPTURE: R-PROCESS Rapid neutron capture or r-process happens when there is a large number of available neutrons • The rate of neutron capture is fast that an unstable nucleus may still be combined with another neutron prior to beta decay (hence the term rapid) NEUTRON CAPTURE: R-PROCESS Rapid neutron capture or r-process happens when there is a large number of available neutrons • Associated with supernovae, in which the temperatures are tremendously high that the neutrons are moving very fast • Neutrons can immediately combine with isotopes that are already heavy PROTON CAPTURE Proton capture or p-process starts with the addition of a p to a nucleus after a supernova is formed The tremendous amount of energy available allows the addition of a p to the nucleus PROTON CAPTURE Proton capture or p-process starts with the addition of a p to a nucleus after a supernova is formed Produces a heavier nucleus that is different from the seed nucleus 1 Stellar nucleosynthesis fusion reactions cannot produce nuclei higher than iron. Synthesis of heavier nuclei happens via neutron or proton capture processes. 2 In neutron capture, a neutron is added to a seed nucleus. The addition of neutron produces a heavier isotope of the element. Neutron capture can occur slowly or rapidly. 3 Beta decay results in an increase in the number of protons of the nucleus by one. Hence, a heavier nucleus is formed. 4 Proton capture or p-process is the addition of a proton in the nucleus. GIVEN THE HALF-LIFE, ASSESS WHETHER THE FOLLOWING NUCLIDES WILL UNDERGO SPROCESS, R-PROCESS, OR DECAY. 1. Fe-70 (77 ms) 2. Sr-88 (stable) 3. Ge-77 (11.21 h) GIVEN THE FIGURE BELOW, WHICH ELEMENTS ARE SYNTHESIZED VIA NUCLEAR FUSION REACTIONS?