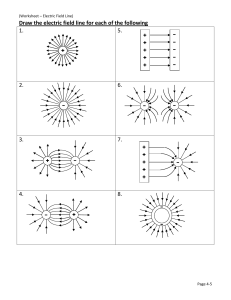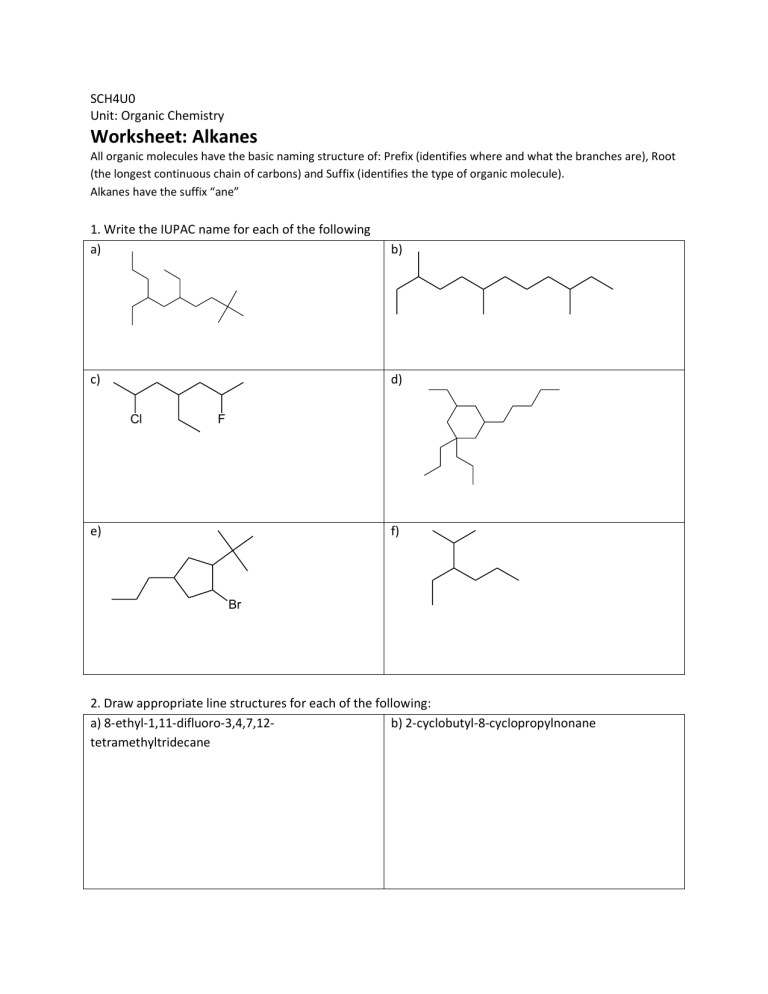
SCH4U0 Unit: Organic Chemistry Worksheet: Alkanes All organic molecules have the basic naming structure of: Prefix (identifies where and what the branches are), Root (the longest continuous chain of carbons) and Suffix (identifies the type of organic molecule). Alkanes have the suffix “ane” 1. Write the IUPAC name for each of the following a) b) c) d) Cl F e) f) Br 2. Draw appropriate line structures for each of the following: a) 8-ethyl-1,11-difluoro-3,4,7,12b) 2-cyclobutyl-8-cyclopropylnonane tetramethyltridecane SCH4U0 Unit: Organic Chemistry Worksheet: Alkenes Alkenes have at least one double bond, which is prioritized (given lowest number) and they have the suffix “ene”. Be aware of the stereoisomerism for double bonds: (E) for branches that are opposite off the double bond, and (Z) for branches that are on the same side of the double bond (this notation goes in the very front of the prefix). 1. Write the IUPAC name for each of the following a) b) F c) d) e) Cl f) Br 2. Draw appropriate line structures for each of the following: a) (2E, 7Z)-8-methyldeca-2,7-diene b) 7-sec-butylcyclohepta-1,3,5-triene SCH4U0 Unit: Organic Chemistry Worksheet: Alkynes The functional group is a triple bond, with a suffix of “yne” 1. Write the IUPAC name for each of the following a) Cl b) Br c) d) e) 2. Draw appropriate line structures for each of the following: a) 3,4,5-triethyl-3-methylhepta-1,6-diyne b) 5-propylundeca-1,3,6,9-tetrayne F R OH SCH4U0 Unit: Organic Chemistry Worksheet: Alcohols The suffix is “ol” 1. Write the IUPAC name for each of the following a) OH b) OH OH c) d) OH OH OH e) f) F OH Cl OH F OH OH 2. Draw appropriate line structures for each of the following: b) 2,3-dichlorocyclopropanol a) 3,6-diethylnon-8-yn-1,3,5-triol O SCH4U0 Unit: Organic Chemistry R H Worksheet: Aldehydes The suffix is “al” 1. Write the IUPAC name for each of the following a) b) O O c) d) O O F Cl O e) O O f) O 2. Draw appropriate line structures for each of the following: a) 3-butyl-4-propylcyclopentanecarbaldehyde b) 2-ethyl-6-hydroxyheptanedial O SCH4U0 R R Unit: Organic Chemistry Worksheet: Ketones The suffix is “one” 1. Write the IUPAC name for each of the following a) b) O O O c) d) O O O O Cl Cl O O e) O 2. Draw appropriate line structures for each of the following: a) 7,10-dimethyltetradec-13-yne-2,5,8,11-tetrone b) 1-phenylbutan-2-one O R SCH4U0 Unit: Organic Chemistry OH Worksheet: Carboxylic Acids The suffix is “oic acid” 1. Write the IUPAC name for each of the following a) b) OH O O O O OH HO HO HO O O c) HO d) Cl HO OH O O e) Br OH O 2. Draw appropriate line structures for each of the following: a) (3E)-8-cyclobutyl-2-cyclopropylnon-3-enedioic b) cyclohexane-1,3,5-tricarboxylic acid acid O R SCH4U0 O R Unit: Organic Chemistry Worksheet: Esters The suffix is “oate”; note that esters are written as two separate parts, the first part does not contain the carbonyl group and has the suffix “yl” 1. Write the IUPAC name for each of the following a) b) O O O O c) d) O O O O O Cl O e) O O 2. Draw appropriate line structures for each of the following: a) prop-2-en-1-yl (9Z)-5,10-difluoroundec-9-en-6b) ethyl 3-butyl-2-ethyl-2ynoate methylcyclopropanecarboxylate R SCH4U0 Unit: Organic Chemistry O R Worksheet: Ethers The suffix is “ane”. Ethers are similar to alkanes in that they don’t have a functional group; the branches end in “oxy” 1. Write the IUPAC name for each of the following a) b) O O O O O c) d) O O O O e) f) O F O O 2. Draw appropriate line structures for each of the following: a) m-dipropoxybenzene b) (3E)-7-(pentyloxy)hept-3-en-1-yne O O SCH4U0 Unit: Organic Chemistry O R R NH2 R N R R NH R Worksheet: Amides The suffix is “amide”. Note that branches attached to the nitrogen are located with an “N”, rather than just a number. 1. Write the IUPAC name for each of the following a) b) NH2 O N O c) d) N NH O O e) f) O H2N Br NH NH O O 2. Draw appropriate line structures for each of the following: a) 3-tert-butyl-N-ethyl-Nb) 9-amino-N,N,5-trimethylundecanamide propylcyclopentanecarboxamide R SCH4U0 Unit: Organic Chemistry R N R NH2 R NH R R Worksheet: Amines The suffix is “amine”. Note that branches attached to the nitrogen are located with an “N”, rather than just a number. 1. Write the IUPAC name for each of the following a) b) HN N NH2 c) H2N NH NH2 e) N d) NH2 f) Cl NH2 O NH NH Cl 2. Draw appropriate line structures for each of the following: a) cyclohept-6-ene-1,2,3,5-tetramine b) (2Z)-N5-ethyl-N8-propylundec-2-en-10-yne-2,5,8triamine SCH4U0 Unit: Organic Chemistry Worksheet: Aromatics The suffix is “benzene”. Note that like all cyclics, the ring (benzene in this case) is generally the root, however it can be treated as a branch and if so the branch name is “phenyl”. 1. Write the IUPAC name for each of the following a) Cl b) Br F c) d) e) f) Cl 2. Draw appropriate line structures for each of the following: a) meta-dibromobenzene b) 3,6,7-triphenyldecane