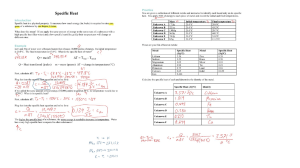
Name: Class: Worksheet: Exothermic and Endothermic Reaction 1. In an exothermic reaction, does the temperature of the surrounding go up or down: 2. In an endothermic reaction, does the temperature go up or down: 3. Examples of exothermic and endothermic reaction: - Ice melting - Combustion - Water evaporation - Photosynthesis - Respiration - Freezing of water - Sports Ice pack Classify these examples into exothermic or endothermic reactions: Exothermic: Endothermic: 4. Circle the correct answers. The bonds between the atoms of the reactants / products need to be broken first, this is an endothermic / exothermic process. The bonds are made between the atoms of the reactants / products, this is an endothermic / exothermic process. 5. Refer to the table to answer these questions. (a) Decide whether each reaction is endothermic or exothermic. Explain how you could tell. (b) Which reaction has the largest energy change? 6. In an exothermic reaction, is enthalpy change positive or negative? 7. In an endothermic reaction, is enthalpy change positive or negative? What is enthalpy? The enthalpy of a reaction is equal to the energy required to break the bonds between reactants minus the energy released by the formation of new bonds in the products. Energy of products: Energy of products: Temperature of surrounding: Temperature of surrounding: Exothermic or endothermic: Exothermic or endothermic: Is energy released or absorbed: Is energy released or absorbed: Make your summary on exothermic and endothermic reaction using these following keywords: enthalpy, equilibrium, absorbed, released, increase, decrease, surrounding, and product When hydrochloric acid reacts with ammonium hydroxide in a beaker, the temperature goes up.