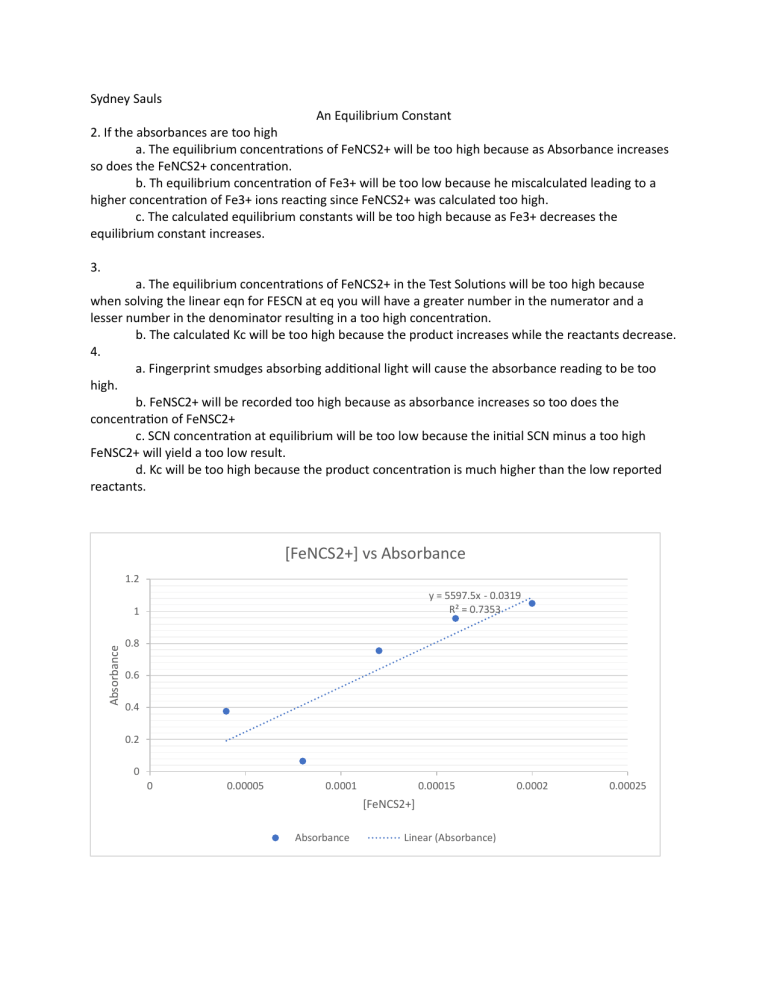
Sydney Sauls An Equilibrium Constant 2. If the absorbances are too high a. The equilibrium concentrations of FeNCS2+ will be too high because as Absorbance increases so does the FeNCS2+ concentration. b. Th equilibrium concentration of Fe3+ will be too low because he miscalculated leading to a higher concentration of Fe3+ ions reacting since FeNCS2+ was calculated too high. c. The calculated equilibrium constants will be too high because as Fe3+ decreases the equilibrium constant increases. 3. a. The equilibrium concentrations of FeNCS2+ in the Test Solutions will be too high because when solving the linear eqn for FESCN at eq you will have a greater number in the numerator and a lesser number in the denominator resulting in a too high concentration. b. The calculated Kc will be too high because the product increases while the reactants decrease. 4. a. Fingerprint smudges absorbing additional light will cause the absorbance reading to be too high. b. FeNSC2+ will be recorded too high because as absorbance increases so too does the concentration of FeNSC2+ c. SCN concentration at equilibrium will be too low because the initial SCN minus a too high FeNSC2+ will yield a too low result. d. Kc will be too high because the product concentration is much higher than the low reported reactants. [FeNCS2+] vs Absorbance 1.2 y = 5597.5x - 0.0319 R² = 0.7353 Absorbance 1 0.8 0.6 0.4 0.2 0 0 0.00005 0.0001 0.00015 [FeNCS2+] Absorbance Linear (Absorbance) 0.0002 0.00025