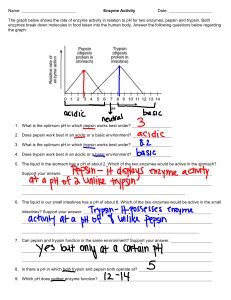
Exploration: An investigation to examine the impact of varying pH on the enzymatic activity of pepsin on ovalbumin Aim: To examine the impact of varying pH on the rate of reaction of pepsin with ovalbumin Research Question: To what extent does pH affect the rate of reaction of pepsin with ovalbumin? This study seeks to examine the extent to which the pH of a solution affects the rate of reaction of pepsin on ovalbumin. The pH will be varied by using different buffer solutions, and its impacts on enzyme activity will be measured by observing the clarity of the pepsin-ovalbumin solution using a colorimeter, where clearer solutions translate into more enzymatic activity. Background Information: One of the more common food pairings known in the western world is a glass of orange juice with a classic breakfast of eggs. The two seem to naturally complement each other, both in terms of taste as well as nutritional value. But there is a scientific theory that could suggest more grounded evidence of orange juice aiding the digestion of eggs itself. Most digestive processes start off with the enzyme-catalyzed breakdown of large macromolecules. Enzymes are globular proteins that consist of amino acids linked by amide and peptide bonds. They are also catalysts of a reaction, meaning they speed up the rate of reaction without being used up in the process themself, and reduce the overall activation energy. Enzymes have an active site on their surface, a location where macromolecules, also known as substrates, can bind to. The enzyme’s active site orientates the substrate such that chemical bonds within the substrate to be more readily broken and formed. Once the reaction is catalyzed, the active site of the enzyme releases the byproduct of the reaction. The surrounding pH of where the enzyme affects the rate of reaction. The rate of digestion of most nutrient molecules is typically highest at a pH of 7, with this being the general pH of the human digestive system, with digestive enzymes working best in a neutral environment. However, it is important to note that there are exceptions to this overview. One of the most notable cases is the pH of the human stomach, which has an acidic pH due to the presence of hydrochloric acid, which kills off pathogens and aids the breakdown of nutrient macromolecules. The enzyme pepsin is also secreted within the stomach and is used to break down proteins into amino acids. One of the many proteins pepsin breaks down is ovalbumin, a storage macromolecule found in the white of chicken eggs. With the pH of orange juice being acidic in nature, with its primary constituent being citric acid, this could hint at a reason behind its potential as an aid to the digestion of egg. The pH of citric acid is around 2.2, a value that falls in the range of the stomach acid pH of 1.5-3.5. It can therefore be inferred that the low pH of orange juice aids the digestion of the ovalbumin components within the egg white due to similarity to the pH of the stomach. With this knowledge in mind, the optimum working pH of the enzyme pepsin can be predicted to be relatively acidic. However, I’d like to conduct an investigation to determine the ideal working pH of the enzyme pepsin acting on ovalbumin, and how this can scientifically justify the food pairing of a breakfast of eggs and orange juice. Hypothesis/ justification: My hypothesis is that as the pH of the pepsin-ovalbumin solution approaches 2, the enzymatic activity will increase, and as the pH level strays further away from 2, the enzymatic activity will subsequently decrease. This is due to the resemblance of this low pH environment to the pH of the stomach. Based on my prior knowledge of how enzymes work, seen in the graph below, I predict that the data will be normally distributed, taking the form of a bell curve when modeled. I intend to use statistical analysis of normal distribution to prove this. (Adam Day, 2018) Selecting Variables: Preliminary Data: Through the conduction of preliminary trials within this experiment, the method of this investigation was established, along with the control variables and dependent variable. The use of a colorimeter, which measures the clarity of a solution was determined to be the most appropriate way to measure enzymatic activity, as the ovalbumin solution would get noticeably clearer when the pH of the solution was close to the optimum. Greenlight (of wavelength 565 nm) was selected as the light to be used as this gave the largest range of % transmittance across all pH levels. Control values were decided based on their practicality, for example, the amount of substance used that could fit in a 20ml test tube along with their resemblance to the conditions of the human body, which was most evident in the value decided for the control temperature (37ºC). Independent variable: The variable that will be changed will be the pH of the enzyme-substrate solution. It will be changed by using pH buffer solutions from a range of 1 to 9, values created by the concentration of hydrogen ions in the solution. Controlled/ Monitored variables: Variable Explanation of why variables need to be controlled. What could happen if it is not? How it is controlled and Value/s Used Temperature Temperature is another factor that affects enzymatic activity, so if it is not controlled, both pH and temperature will affect the results of the data Test tubes containing enzyme-solution mixture will be kept in a hot water bath set at 37ºC Substrate Concentration The amount of substrate molecules per unit of solution will affect the number of interactions between the enzymes and substrate, due to the nature of enzyme-substrate collisions, and will thus create inconsistencies in measurements of enzyme activity 15 grams of ovalbumin powder per 100 ml of water will be the concentration of the substrate Enzyme Concentration Enzyme activity is dependent on the number of enzymes per unit of the solution, and different concentrations of the enzyme will result in differing values of enzymatic activity 5% pepsin solution will be used Time taken for the reaction to elapse More time that is given for an enzyme-substrate interaction to occur means that higher levels of enzymatic activity could be recorded The enzyme will be added to the substrate for 10 minutes Volume of Enzyme Different amounts of pepsin would result in different readings taken for their reactions due to the nature of more particles resulting in more reactions occurring. 5ml of pepsin solution will be added to each test tube Volume of Substrate Different amounts of ovalbumin would result in different readings taken for their reactions due to the nature of more particles resulting in more reactions occurring. 5ml of ovalbumin solution will be added to each test tube The volume of pH buffer solution The volume of pH also needs to stay constant in order to have the desired impact on the pH of the solution. 5ml of pH buffer will be added to each test tube Number of stirs when mixing stock solution Differing amounts of stirs when mixing ovalbumin with water will result in different amounts of ovalbumin dissolved per unit of water, which could result in more or less enzymatic activity and different More than 100 stirs will be used to mix ovalbumin and water together Time taken for substrate solution to heat up The substrate requires time to heat up to reach the enzyme’s optimum working temperature, in order to provide the environment for the maximum enzymatic activity to occur A stock solution of ovalbumin and water should be kept in a hot water bath of 37ºC for 10 minutes before mixing with pepsin and should be verified by direct measurement using the thermometer Time taken for the enzyme to heat up The enzyme requires time to heat up to its optimum working temperature, and the optimum working temperature must be reached in order to keep the level of enzymatic activity constant Pepsin should be kept in the hot water bath of 37ºC for 10 minutes before mixing with ovalbumin solution Dependent Variable: Enzymatic activity will be measured by observing the clarity of the solution after 10 minutes of pepsin-ovalbumin interaction. A colorimeter will be used to measure the transparency of the solution, which shines a light of fixed wavelength through a cuvette containing solution, and measures the percentage of light that is transmitted through the cuvette. The clearer the solution, the more ovalbumin the pepsin has broken down, showing more enzymatic activity. Apparatus: Apparatus Quantity Size / concentration/ Volume Accuracy Syringes 11 Size 10ml ±0.1 ml Spatula 1 Standard Size ±0.01g Safety Goggles Top Pan Balance 1 Stopwatch 1 Pepsin Solution 1 Computer with Logger Pro Software 1 ovalbumin Powder 1 50g Distilled Water 1 250ml Test Tubes 45 20ml Hot Water Bath 1 Set to 37ºC ±0.1ºC Colorimeter Equipment (+ USB to USBC Adapter) 1 Set to 565nm (green light) ± 25 mV Thermometer 1 100 ±0.1ºC Cuvette 18 3.5ml ±0.3s 250ml of 5% concentration Gloves Glass Stirring Rod 1 pH buffer solutions 9 pH from 1-9 Diagram: Methods: 1. Create a stock solution of ovalbumin and water, using 45 grams of ovalbumin powder and 300 ml water, using a top-pan balance + spatula and measuring cylinder (100ml) respectively 2. Fill hot water bath to 50% capacity, and set the temperature to 37ºC 3. Set up the colorimeter, plugging it into a computer using logger pro to record data 4. Calibrate the colorimeter using the stock solution, using a wavelength of 565 nm (green light) 5. Distribute 5ml stock solution into 9 test tubes using a 10ml syringe 6. Wear gloves and safety goggles. 7. Label 9 test tubes numbers 1 through 9 8. Add a 5ml of pH buffer solution to each test tube, ranging from pH 1-9, using a different syringe for each pH, and place in the hot water bath (37ºC) for 10 mins alongside the beaker of pepsin solution 9. Mix 5ml pepsin solution into each test tube containing ovalbumin-pH buffer solution whilst inside the hot water bath, and start the stopwatch 10. After ten minutes has elapsed, transfer the contents of each test tube into a cuvette, labeling each cuvette the number of its respective test tube, from 1-9 11. Place cuvette inside colorimeter and establish the transmittance of each solution by starting the logger pro programme 12. Record the results from the colorimeter after transmittance has stabilized, and repeat steps 4-11 four more times Sufficient & Reliable Data: The enzyme will be varied by using pH buffer solutions from a range of 1-9, in order to establish the optimum working pH of the solution. I have chosen this range as my hypothesis predicts that the optimum pH of pepsin lies close to 2 or 3, and have involved 5 trials for each in order to establish a reliable mean transmittance of light through each solution. Safety, Ethical and Environment Considerations: The use of a hot water bath could potentially be dangerous. The level of water in the hot water bath should not exceed half the length of the test tube, and the hot water bath should be opened in the direction opposing the participant’s body. The acidic and alkaline pH buffer solutions could also be a potential irritant, meaning that gloves should be used when extracting them from their containers using a syringe. Safety goggles should be used at all times to minimize eye exposure to pH buffers and water vapor. The laptop and colorimeter equipment used should be kept away from the test tubes and hot water to minimize damage from water vapor and water from the hot water bath. To minimize environmental implications, enzymes, substrate, and pH buffer mixture should be disposed of in a waste disposal container, as opposed to pouring down the sink. The waste should also be minimised by using small volumes of substrate, pH buffer, and enzyme solution. Analysis Data table(s) Table 2: Processed data showing average transmittance of green light through solution and Table 1: Raw data showing the clarity of enzyme-substrate solutions after five trials pH of buffer solution % Transmittance of Green Light Through Cuvette ±0.7 pH of buffer solution ±0.5 t1 Calibrated reading with stock solution t2 t3 t4 Average Transmittan ce of light through Standard solution (%) Deviation t5 1 75 4 42 42 42 42 42 2 99 0 1 73 70 76 78 80 3 82 4 2 99 99 99 99 98 4 62 2 3 86 87 80 75 83 5 55 2 4 61 64 66 59 62 6 51 3 5 52 58 56 54 55 7 47 2 6 48 50 55 48 53 8 46 2 7 45 47 50 44 48 9 45 2 8 44 44 50 46 45 9 44 44 48 44 44 Data processing Table 2 Row 1 sample calculations: Average transmittance of light through solution=(t1+t2+t3+t4+t5)/5=(73+70+76+78+80)/5=75 Standard deviation = STDEV (73, 70, 76, 78, 80) = 4 Qualitative Data At pHs 2 and 3, a white precipitate was observed at the bottom of the test tube containing the substrate, ph buffer solution and enzyme after heating, and the liquid of the mixture was significantly more transparent than higher pH solutions, in which no real difference could be observed from the original stock solution. Graphs: Fig. 1: A line graph to show the effect of pH on the mean transmittance of green light through a pepsin-ovalbumin solution: Fig 2: A Gaussian curve showing the effect of pH on the mean transmittance of green light through a pepsin-ovalbumin solution Statistics: selection and support In order to use statistics to justify my claims, I will use a null and alternative hypothesis to test for a relationship between pH and enzymatic activity: H0 (null hypothesis) - there is no distinction in enzymatic activity present between solutions of differing pHs H1 (alternative hypothesis) - high amounts of enzymatic activity will be concentrated around a specific pH value, indicating an optimum working pH of the solution The data gathered in Fig. 1 has been processed using a gaussian curve, as seen in Fig. 2, which tests the normal distribution of data along a series of intervals. This is usually represented by a histogram, where the frequency of occurrence can be represented over fixed intervals, but seeing as there are fixed data points for this experiment, a bell curve can be drawn by using individual data points. For the normal distribution, the bell curve tends to peak around the mean value of the independent variable and indicates dependent values above and below the mean by using positive and negative standard deviation values. The original null hypothesis stated that there is no distinction in enzymatic activity present between solutions of differing pHs, and the alternative hypothesis stated that high amounts of enzymatic activity will be concentrated around a specific pH, indicating a normal distribution. n this experiment, there were 9 data points and a correlation coefficient of 0.9883, as calculated by the logger pro software. This means that the data received from the experiment is very accurate, and it aligns with the findings of previous studies about the distribution of enzymatic activity, with enzymatic activity always being modeled using a bell/gaussian curve. The low RMSE (root mean square error) value- a measurement to detect the variation of observed data with respect to a model- of 3.65, calculated by the logger pro software compared to the range of the readings taken (55) also gives credibility to the data and its processing. In conclusion, the null hypothesis can be rejected and the alternative hypothesis can be accepted, as the data fits the Gaussian curve to very high accuracy and there is a clear relationship between pH and enzyme activity evident. Evaluation Research Question My original research question was: to what extent does pH affect the rate of reaction of pepsin with ovalbumin? My hypothesis based on this question was that as pH approached a value of 2, the enzymatic activity would increase, and pH values higher than or lower than 2 would result in reduced enzymatic activity. Discussion: Looking at Fig. 1, a line graph, there is evidence to suggest that the enzymatic activity of pepsin is highest at pH 2. However, this is largely due to the fact that the line graph is simply a connection between the data points received in the experiment. After processing the data by looking at its normal distribution in Fig. 2, it becomes clear that the highest distribution of enzymatic activity occurs at a pH of approximately 2.2, and the rest of the data is distributed symmetrically in the form of a bell curve around this point from pHs 1-5. However, at pHs beyond 6, there seems to be little to no effect of varying pH on the enzymatic activity of pepsin. Conclusion: With my original hypothesis predicting that the optimum working pH of pepsin would be 2, and the value obtained from the experiment giving an optimum pH of 2.2, this hypothesis can be accepted with relative confidence. With regards to my research question concerning the extent to which pH affects the rate of reaction of pepsin on ovalbumin, I can safely conclude that pH has a huge effect on the rate of reaction of pepsin on ovalbumin, which was quantitatively measured by observing the clarity of the enzyme-substrate solution, with clearer solution meaning more enzymatic activity. At more acidic pHs, the rate of reaction was highest, whilst at more neutral and basic pHs, there was almost no enzymatic activity occurring, with the clarity of the solutions being near identical to the clarity of the ovalbumin stock solution of 42%. The qualitative results observed in this experiment also align with the quantitative results gathered, as the breakdown of the ovalbumin solution into clear water and white amino acids for pHs 2 and 3 show the optimal acidity of the solution. Numerous figures give great credibility to the experiment conducted; the first being the small error bars generated from low standard deviation values, which emphasize the data being consistent throughout the numerous trials conducted. The second indicators of good are produced by the Gaussian curve used to analyze the data, where the high correlation coefficient (a calculation that maps out how well the data fits the model created) of 0.9883, and the low RMSE (a value that calculates how far the data strays away from the model on average) value shows a narrow range indicating more reliable results. The final conclusions of this experiment also support the original thinking of the optimum pH of pepsin. Explanation: From the data collected from the experiment and the Gaussian curve used to analyze it, it becomes clear that the optimum working pH of pepsin on ovalbumin is 2.2. All of the data points lie very close to the curve, with zero anomalies, and the size of the error bars indicates low variability within the experiment. As previously mentioned, this has to do with the location where different substrates are digested within the human body, and in this particular case, ovalbumin, the protein compound found in chicken eggs, is digested within the stomach, which has an acidic pH in order to make food more readily digestible and to exterminate any pathogens that could pose a threat to the body. In order for pepsin to digest the proteins in this environment, it must have an optimum working pH similar to that of the stomach pH, which lies in between 1.5-3.5. Fig. 3- Pepsin Structure (Cooper 1990) Fig. 4- Ovalbumin Structure (Stein 1991) Pepsin’s effectiveness to work in such a low pH is completely reliant on its molecular structure. Pepsin’s original form is pepsinogen, an inactive enzyme that becomes activated when in contact with stomach gastric acid. As mentioned previously, pepsin is a globular protein that is formed by linking a series of amino acids. The structure of any protein is dictated by the amino acid sequence, the interactions of adjacent hydrogen bonds, and the chemical reactions that occur in 2d and 3d forms. With pH being defined as -log10c, where c is the concentration of hydrogen ions in moles/liter, it becomes clear that pH does in fact have a role with regards to the chemical reactions that take place. The carboxylic acid group on the amino acid located on pepsin’s active site needs to be in a protonated state, meaning that it is bound to a hydrogen atom. This protonation is maintained at low pHs, but at high pHs, the carboxylic acid becomes deprotonated, meaning that pepsin no longer has the ability to act in chemical reactions with substrates (Updegraff). It is established that at pH 2 pepsin is most active and that beyond a pH of 6.5, pepsin is rendered completely inactive, matching the findings of the experiment I conducted. Along with deprotonation, which inhibits the enzyme’s active site, high pHs also result in the denaturation of the entire enzyme as a whole, where pepsin (Fig. 3) folds in on itself as part of the interactions of a solution with a more positive charge (higher pH). However, up until pHs of 8.5, pepsin still has the ability to become active once more, where it must overcome the folding barrier previously described (Cooper 222). With regards to the relationship between the substrate and the enzyme used, pepsin falls into the larger enzyme category of proteases, which digest proteins within the human body. Ovalbumin is a storage glycoprotein located within the chicken egg white and is useful to humans by providing a source of protein for muscle growth and repair whilst being low in cholesterol, calories, and lipid concentration. Whilst the structure of ovalbumin is relatively unknown, it is thought to be a connection between two peptide chains (Fig. 4), which are broken down by pepsin in a hydrolysis reaction into independent peptide chains to be used in the human body (Abeyrathne 2686). The optimum pH of pepsin determined in this experiment closely matches the claims made by previous research, where the highest enzymatic activity occurred at more acidic pHs of 2. This answers my original question of the extent to which pH affects the rate of reaction of pepsin, which I now know is to a very large extent. It also addresses the science behind my interest in this topic originally, where I discovered that the citric acid of orange juice can act as a buffer to reduce the pH of the environment that ovalbumin is digested in, increasing the rate of reaction of pepsin. Evaluation: Strengths & Limitations This experiment underwent 5 trials per different pH buffer solution, meaning that the uncertainty taken into account from random errors affecting each trial is minimized overall. The use of many control variables, such as amount of substance used in each trial, as well as the temperature controls and substrate concentration in each mixture, which are two factors which are known to impact enzymatic activity. Weaknesses and Improvements Limitation/ Weakness Significance of error Improvement The ratio of ovalbumin to water in-stock solution There wasn’t enough albumin within the stock solution, meaning that at the optimum pH of 2.2, nearly all of the ovalbumin had been digested, producing an almost perfectly transparent solution with a transmittance of 99%. This could act as an artificial ceiling to the findings of the experiment and may cause errors in the model used to determine the optimum pH Increase ratio of albumin to water from 15g:100ml to 25g:100ml Lack of stirring of stock solution and pH buffer Whilst the stock solution was adequately stirred in order to create a consistent solution, the lack of the mixing of the stock solution and pH buffer may cause some portions of the mixture to have differing pHs, which would result in inconsistent levels of enzymatic activity recorded across the solution Mix the stock solution and the pH buffer before adding the enzyme using a glass stirring rod Lack of stirring of pepsin and ovalbumin stock solution Whilst the stock solution was adequately stirred in order to create a consistent solution, pepsin would only work at the point of contact of the two solutions, and there would be sections of the mixture that would have zero contact with the enzyme, resulting in inconsistencies in the enzymatic activity measured Mix the ovalbumin stock solution and pepsin solution using a glass stirring rod before heating Failure to measure pH of ovalbumin, pepsin, and pH buffer solution Whilst the pH buffer solution prepared gave some indication as to the pH of the solution, the addition of pepsin and albumin to the pH buffer could’ve potentially altered the pH of the mixture as a whole, giving false readings for the independent variable recorded from each data set Test whole solution after heating by using a pH probe to establish true pH value Use of same ovalbumin stock solution through the whole experiment Any error created by measuring quantities of ovalbumin powder and water would’ve been perpetuated through all of the trials, creating errors in data gathering Create 5 separate stock solutions for each round of trials, to ensure systematic errors created by mistakes in preparation of stock solution is minimized Extension: One of the extensions that would improve the quality of this experiment would be increasing the number of intervals used when varying pH. Whilst the model created when analyzing the raw data from the graph gives an optimum working pH of 2.2 for pepsin, which is consistent with previous findings, by using intervals such as 0.2 instead of 1 for varying the pH, a more accurate optimum pH could be observed by using a similar gaussian test for normal distribution, Another investigation that could act as an extension of these findings could be observing the effect of changing pH on the enzymatic activity of gastric lipase, an enzyme also found in the stomach that targets lipids, and observing the relationship between the optimum working pH of pepsin and gastric lipase. Seeing as quite a wide range of data was collected from varying the pH, from transmittances of 45 to 99, it would be interesting to see the effect of varying the surrounding temperature of pepsin, and observe whether pH or temperature alters the rate of reaction more. Citations Abeyrathne E.D.N.S. , Lee H.Y., Jo C., Nam K.C., Ahn D.U., Enzymatic hydrolysis of ovalbumin and the functional properties of the hydrolysates, Poultry Science, Volume 93, Issue 10, 2014, Pages 2678-2686, Castellano AC, Barteri M, Bianconi A, Bruni F, Della Longa S, Paolinelli C. Conformational changes involved in the switch from ovalbumin to S-ovalbumin. Z Naturforsch C. 1996 May-Jun;51(5-6):379-85. PMID:8663899 Cooper JB, Khan G, Taylor G, Tickle IJ, Blundell TL. X-ray analyses of aspartic proteinases. II. Three-dimensional structure of the hexagonal crystal form of porcine pepsin at 2.3 A resolution. J Mol Biol. 1990 Jul 5;214(1):199-222. PMID:2115088 Engineering ToolBox, (2003). Acids - pH Values. [online] Available at: https://www.engineeringtoolbox.com/acids-ph-d_401.html [Accessed Day Mo. Year]. Khan Academy. "Enzyme reaction velocity and pH | Cellular energetics | AP Biology | Khan Academy." YouTube, 24 Oct. 2018, www.youtube.com/watch?v=LtGCHzKAf8w. Accessed 10 May 2021. Meek RL, Walsh KA, Palmiter RD. The signal sequence of ovalbumin is located near the NH2 terminus. J Biol Chem. 1982 Oct 25;257(20):12245-51. PMID:6749856 Stein, P.E., Leslie, A.G., Finch, J.T., Carrell, R.W. (1991) J Mol Biol 221: 941-959 DOI: 10.1016/0022-2836(91)80185-w Updegraff, Emily. "What Is the Optimum PH for Human Stomach Enzyme Activity?" Sciencing, 25 June 2018, sciencing.com/what-is-the-optimum-ph-for-human-stomach-enzyme-activity-12072183 .html. Accessed 7 May 2021.