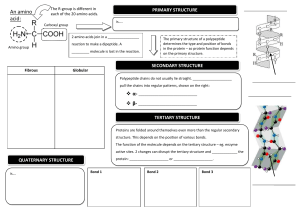
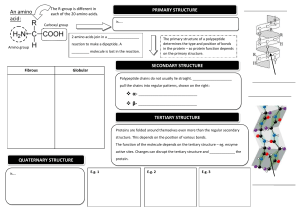
Pre-class textbook reading for Friday, January 5th Note – although Bio125 does not have a college chemistry prerequisite, we will rely on your previous familiarity with chemical bonds and structures. The following reading provides some excellent background and review to help you understand material that will be presented this term. Bioskills 14 “Reading Chemical Structures” (the Bioskills section are immediately after chapter 1) Review the four methods of displaying the chemical structure of a molecule as illustrated in Figure B14.1 From Chapter 2: Beginning of chapter through “polar bonds produce …”; section 2.2 but stop before “cohesion, adhesion, and surface tension” I have chosen a few pages from this chapter in order to remind you of some fundamental features associated with chemical structure. Here are some key points that you should understand: Atomic structure, figure 2.1a (protons, neutrons, electrons) Mass number vs atomic number, figure 2.2 - how is the periodic table organized? Covalent chemical bonds – how are they formed? Figure 2.5b – why does the oxygen atom in the water molecule have a slight negative charge? What gives a water molecule its polar characteristics? What is a hydrogen bond? (We will see this type of chemical bond frequently in this course!) Hydrophilic vs. hydrophobic From Chapter 3: entire chapter Proteins are such integral players in biological processes that you should familiarize yourself with their structure and function. Key points from the chapter: Figure 3.1a – what groups are attached to the central carbon atom of an amino acid? Variation in the 20 amino acids resides in the R-group (figure 3.2 should help you appreciate the variability, but do not try to memorize!!!). The peptide bond and the chemical reaction linking two amino acids (figure 3.3) Amino vs. Carboxyl ends of a protein (polypeptide) Protein primary structure. Check out the example in figure 3.7 in which a single amino acid change can have dramatic functional consequences for the protein. Protein secondary structure (figure 3.8). What are the molecular interactions that permit a region of alpha helix to form within a protein sequence? (Look up the definition of Hydrogen-bonding). Tertiary and Quaternary structure Protein folding is crucial for the proper functioning of a protein What is an “enzyme”? From Chapter 4: beginning of chapter, sections 4.1 and 4.2. For a course that is centered around “biological information”, it does not get more exciting than studying the nucleic acids. Enjoy reading about DNA and RNA since these molecules will be your life-long friends! Some key concepts from the chapter that you will see often throughout the term: From figure 4.1: What are the three components of a nucleotide, what are the structural difference between ribose vs deoxyribose sugar, how do RNA and DNA differ in their nitrogenous bases? Nucleic acids are polymers of nucleotides linked together in a chain. Formation of the phosphodiester bond that links nucleotides together to produce the sugar-phosphate backbone of the molecule (figures 4.2 and 4.3) Directionality of a nucleic acid molecule (5’ and 3’ ends) The secondary structure of DNA (i.e. the double helix) is crucial for the molecule’s function and stability. Be sure to understand the following: complementary base pairing rules, antiparallel orientation of the two strands, hydrogen bonding between the base pairs, shorthand notation of DNA sequence. (Study figures 4.5 and 4.6)