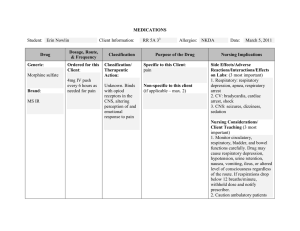
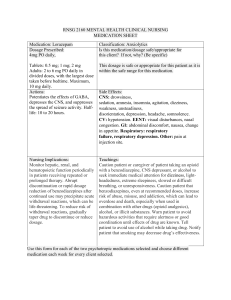
Drug Class Drug Sample Pharmacodynamics Potentiates the effects of GABA, depresses the CNS, and suppresses the spread of seizure activity. Anxiety Acute alcohol withdrawal Before endoscopic procedures Muscle spasm Preoperative sedation Adjunctive treatment for seizure disorders Status epilepticus, severe recurrent seizures Patients on stable regimens of antiepileptic drugs who need diazepam intermittently to control bouts of increased seizure activity Tetanus Acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from usual seizure pattern in patients with epilepsy Potentiates the effects of GABA, depresses the CNS, and suppresses the spread of seizure activity. Anxiety Insomnia from anxiety or transient situational stress Preoperative sedation Status epilepticus Diazepam Benzodiazepines Lorazepam CENTRAL NERVOUS SYSTEM (CNS) DRUGS ANTIEPILEPTIC ( ANTICONVULSANTS ) Indications Side Effects CNS: drowsiness, dysarthria, slurred speech, tremor, transient amnesia, fatigue, ataxia, headache, insomnia, paradoxical anxiety, hallucinations, minor changes in EEG patterns, pain, vertigo, confusion, depression. CV: CV collapse, bradycardia, hypotension. EENT: diplopia, blurred vision, nystagmus; with nasal form: nasal discomfort, nasal congestion, epistaxis. GI: nausea, constipation, diarrhea with rectal form, dry mouth, dysgeusia (nasal form). GU: incontinence, urine retention. Hematologic: neutropenia. Hepatic: jaundice. Respiratory: respiratory depression, apnea, hiccups. Skin: rash, phlebitis at injection site. Other: altered libido, physical or psychological dependence. CNS: drowsiness, sedation, amnesia, insomnia, agitation, dizziness, weakness, unsteadiness, disorientation, depression, headache, somnolence. CV: hypotension. EENT: visual disturbances, nasal congestion. GI: abdominal discomfort, nausea, change in appetite. Respiratory: respiratory failure, respiratory depression. Other: pain at injection site. Nursing Considerations • Periodically monitor LFTs, CBC, and renal function in patients receiving repeated or prolonged therapy. • Monitor HR, BP, and mental status changes. Patients are at an increased risk for falls. Alert: Use of drug may lead to abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines frequently involves concomitant use of other medications, alcohol, or illicit substances, which increase frequency of serious adverse outcomes. Assess each patient’s risk of abuse, misuse, and addiction before prescribing and throughout treatment. • Look alike–sound alike: Don’t confuse diazepam with diazoxide or Ditropan. Don’t confuse Valium with Valcyte. • Monitor hepatic, renal, and hematopoietic function periodically in patients receiving repeated or prolonged therapy. To reduce risk of withdrawal reactions, gradually taper drug to discontinue or reduce dosage. • Look alike–sound alike: Don’t confuse lorazepam with alprazolam, clonazepam, or Lovaza. Don’t confuse Ativan with Atgam. Benzodiazepines Barbiturates Unknown. Probably acts by facilitating the effects of the inhibitory neurotransmitter GABA. Lennox-Gastaut syndrome, atypical absence seizures, akinetic and myoclonic seizures Panic disorder Produces all levels of CNS depression. Depresses the sensory cortex, decreases motor activity, and alters cerebellar function. Inhibits transmission in the nervous system and Treatment of all types of seizures except absence seizures Anticonvulsant in tonic-clonic (grand mal), partial, and febrile seizures in children Preoperative sedative and in other situations in which sedation may be required Clonazepam Phenobarbital CNS: amnesia, aphonia, choreiform movements, coma, confusion, depression, dysarthria, dysdiadochokinesis, hallucinations, headache, hemiparesis, hypotonia, hysteria, increased libido, insomnia, psychosis, slurred speech, tremor, vertigo, paradoxical reactions CV: palpitations. EENT: abnormal eye movements, diplopia, nystagmus, blurred vision, pharyngitis, rhinitis, sinusitis. GI: anorexia, coated tongue, constipation, diarrhea, dry mouth, encopresis, gastritis, nausea, sore gums GU: dysuria, enuresis, nocturia, urine retention, colpitis, dysmenorrhea, delayed ejaculation, erectile dysfunction, urinary frequency Hematologic: anemia, eosinophilia, leukopenia, thrombocytopenia. Hepatic: hepatomegaly, transient elevations of serum transaminases and ALP. Respiratory: chest congestion, hypersecretion in upper respiratory tract passages, respiratory depression, rhinorrhea, shortness of breath, bronchitis, URI, cough. Skin: hair loss, hirsutism, rash. CNS: hangover, delirium, depression, drowsiness, excitation, lethargy, vertigo. Respiratory: respiratory depression CV: hypotension. GI: constipation, diarrhea, nausea, vomiting. Skin: photosensitivity, rashes, Alert: Closely monitor all patients for changes in behavior that may indicate worsening of suicidal thoughts or behavior or depression. • Don’t stop drug abruptly because this may worsen seizures. Notify prescriber at once if adverse reactions develop. • Closely assess response of older adults, who are more sensitive to drug’s CNS effects. • Monitor patient for oversedation. • Monitor CBC and LFTs. • Withdrawal symptoms are similar to those of barbiturates. • To reduce inconvenience of somnolence when drug is used for panic disorder, giving one dose at bedtime may be desirable. • Look alike–sound alike: Don’t confuse clonazepam with clonidine, clozapine, or lorazepam. Don’t confuse Klonopin with clonidine. Monitor phenobarbital concentrations, CNS status, and CBC with differential Monitor signs of hypersensitivity reactions. Notify physician immediately because these symptoms may indicate serious reactions. Assess symptoms of bronchospasm and laryngospasm. Perform pulmonary function tests to document suspected changes in ventilation and respiratory function. Secobarbital Phenytoin Hydantoin (Dilantin) raises the seizure threshold. Capable of inducing (speeding up) enzymes in the liver that metabolize drugs, bilirubin, and other compounds. Hypnotic (short-term) urticaria. MS: arthralgia, myalgia, neuralgia. Others: hypersensitivity reactions, physical dependence, psychological dependence. Produces CNS depression through gammaaminobutyric acid (GABA)–like effects. Therapeutic Effects: Induction of sleep, sedation, or anesthesia. Short-term treatment of insomnia Adjunctive agent for anesthesia May stabilize neuronal membranes and limit seizure activity either by increasing efflux or decreasing influx of sodium ions across cell membranes in the motor cortex during generation of nerve impulses. To control tonic-clonic (grand mal) and psychomotor (temporal lobe) seizures To control tonic-clonic (grand mal) and psychomotor (temporal lobe) seizures in patients requiring a loading dose Generalized tonic-clonic status epilepticus; prevention and treatment of seizures during neurosurgery CNS: abnormal thinking, behavior changes, delirium, excess sedation, excitation, hallucinations, mental depression, vertigo, sleep disorders, sleep—driving, neuralgia, "hangover" effect Respiratory: respiratory depression. GI: nausea, vomiting, constipation Skin: photosensitivity, rashes, urticaria MS: arthralgia, myalgia. Others: physical dependence, psychologic dependence. CNS: ataxia, decreased coordination, mental confusion, slurred speech, dizziness, headache, insomnia, nervousness, twitching, peripheral neuropathy, vertigo. CV: bradycardia, periarteritis nodosa, hypotension, CV shock EENT: diplopia, nystagmus, blurred vision, thickening of facial features. GI: gingival hyperplasia, nausea, vomiting, constipation. Hematologic: agranulocytosis, leukopenia, , thrombocytopenia, macrocythemia, megaloblastic anemia, pancytopenia Hepatic: toxic hepatitis. Metabolic: hyperglycemia. Musculoskeletal: osteomalacia. Monitor daytime drowsiness and ―hangover‖ symptoms Assess seizures Be alert for depression, delirium, excitation, or other alterations in mood or behavior. Assess BP after IV administration and compare to normal values. Report hypotension, especially if patient experiences dizziness or syncope. Assess symptoms of respiratory depression. Monitor pulse oximetry and perform pulmonary function tests to quantify suspected changes in ventilation and respiratory function. Monitor daytime drowsiness. Assess dizziness and vertigo that might affect gait, balance, and other functional activities Report any personality and behavioral changes, including excitation, hallucinations, mental depression, delirium, or expression of abnormal thoughts. Assess any muscle, joint, or nerve pain to rule out musculoskeletal pathology; that is, try to determine if pain is drug induced rather than caused by biomechanical or neurophysiologic problems. • If rash appears, stop drug. If rash is scarlatiniform or morbilliform, resume drug after rash clears. If rash reappears, stop therapy. If rash is exfoliative, purpuric, or bullous, don’t resume drug. • Don’t stop drug suddenly because this may worsen seizures. Call prescriber immediately if adverse reactions develop. • Monitor drug level. Therapeutic level of total phenytoin is 10 to 20 mcg/mL in adults and children and 8 to 15 mcg/mL in neonates. Therapeutic range of free phenytoin is 1 to 2 mcg/mL. • Long-term use may decrease bone mineral density. Vitamin D and calcium supplements may be needed. • Because of the risks of cardiac and local toxicity with parenteral phenytoin, use oral form when possible. • Monitor CBC and calcium level every 6 months, and periodically monitor hepatic function. If megaloblastic anemia is evident, prescriber may order folic acid and vitamin B12 • Maintain seizure precautions, as needed. Alert: Closely monitor all patients for changes in behavior that may indicate worsening of suicidal Skin: SJS, toxic epidermal necrolysis, bullous or purpuric dermatitis, exfoliative dermatitis, hypertrichosis, inflammation at injection site, necrosis, pain, photosensitivity reactions, scarlatiniform or morbilliform rash. Elevates the seizure threshold. Suppresses abnormal wave and spike activity associated with absence (petit mal) seizures. Absence seizures (petit mal) Hasn't been established. Activity in epilepsy is thought to be related to increased brain concentrations of GABA. Simple and complex absence seizures, mixed seizure types (including absence seizures) Complex partial seizures Mania To prevent migraine headache Ethosuximide Valproic Acid CNS: increased frequency of tonic-clonic seizures, dizziness, ataxia, drowsiness, euphoria, fatigue, headache, hyperactivity, irritability, psychiatric disturbances. EENT: myopia. GI: abdominal pain, anorexia, cramping, diarrhea, nausea, vomiting, weight loss, hiccups. GU: pink/brown discoloration of urine, vaginal bleeding. Skin: Stevens-Johnson syndrome, hirsutism, rashes, urticaria. Hematologic: agranulocytosis, eosinophilia, leukopenia, pancytopenia. Other: systemic lupus erythematosus. CNS: asthenia, dizziness, headache, insomnia, paresthesia, tardive dyskinesia, nervousness, somnolence, vertigo, tremor, agitation, abnormal gait, dysarthria, hallucinations, hypertonia, amnesia, ataxia, depression, speech disorder. CV: chest pain, edema, HTN, hypotension, vasodilation, tachycardia, palpitations. EENT: blurred vision, diplopia, conjunctivitis, eye pain, thoughts or behavior or depression. • Watch for gingival hyperplasia, especially in children. Alert: Doubling the dose doesn’t double the level but may cause toxicity. Consult pharmacist for specific dosing recommendations. • If seizure control is established with divided doses, once-daily dosing may be considered. • Look alike–sound alike: Don’t confuse phenytoin with mephenytoin, fosphenytoin, phenelzine, phentermine, or phenobarbital. Don’t confuse Dilantin with Dilaudid, diltiazem, or Dipentum. Document the number, duration, and severity of seizures to help determine if this drug is effective in reducing seizure activity. Monitor skin reactions such as rash, itching/burning skin, hives, exfoliation, and dermatitis. Be alert for signs of agranulocytosis and leukopenia, eosinophilia, or fatigue and poor health that might be due to other blood dyscrasias. Assess dizziness or ataxia that might affect gait, balance, and other functional activities. Monitor daytime drowsiness, euphoria, irritability, or other psychiatric disturbances. Monitor signs of drug-induced lupus syndrome. Periodically assess body weight and other anthropometric measures. • When converting adults and children age 10 and older with seizures from Depakote to Depakote ER, make sure the extended-release dose is 8% to 20% higher than the regular dose taken previously. • Never withdraw drug suddenly because sudden withdrawal may worsen seizures. • Notify prescriber if tremors occur • Monitor drug level. • When converting patients from a brand-name drug to a generic drug, use caution because breakthrough seizures may occur. Alert: Sometimes fatal, hyperammonemic encephalopathy may occur when starting valproate therapy in patients with UCD. Carbamazepine Thought to stabilize neuronal membranes and limit seizure activity by either increasing efflux or decreasing influx of sodium ions across cell membranes in the motor cortex during generation of nerve impulses. Generalized tonic-clonic and complex partial seizures, mixed seizure patterns Acute manic and mixed episodes associated with bipolar I disorder Trigeminal neuralgia nystagmus, deafness, ear pain, pharyngitis, periodontal abscess, rhinitis, tinnitus. GI: abdominal pain, anorexia, diarrhea, fecal incontinence, flatulence, gastroenteritis, glossitis, stomatitis, dyspepsia, hematemesis, eructation, pancreatitis, constipation GU: vaginitis, dysmenorrhea, dysuria, cystitis, urinary frequency Hematologic: hemorrhage, thrombocytopenia Musculoskeletal: back and neck pain, arthralgia, leg cramps, twitching, myasthenia. Respiratory: bronchitis, dyspnea Skin: alopecia, ecchymosis, petechiae, discoid lupus erythematosus, furunculosis, seborrhea, rash, pruritus CNS: ataxia, dizziness, drowsiness, somnolence, vertigo, worsening of seizures, confusion, fatigue, fever, headache, syncope, pain, depression, speech disorder. CV: arrhythmias, AV block, HF, HTN, hypotension. GI: nausea, vomiting, abdominal pain, anorexia, diarrhea, dry mouth, dyspepsia, glossitis, stomatitis. Hematologic: agranulocytosis, aplastic anemia, leucocytosis, thrombocytopenia, eosinophilia, Respiratory: pulmonary hypersensitivity. Skin: erythema multiforme, SJS, toxic epidermal necrolysis, excessive diaphoresis, rash, urticaria, pruritus. Alert: Closely monitor all patients taking or starting antiepileptic drugs for changes in behavior indicating worsening of suicidal thoughts or behavior or depression. Alert: Dose-related thrombocytopenia can occur. Monitor CBC, platelet count, PT, and INR before starting therapy and at frequent intervals. Alert: Monitor patients and immediately report symptoms of DRESS syndrome • Look alike–sound alike: Don't confuse Depakote with Depakote ER. • Watch for worsening of seizures, especially in patients with mixed seizure disorders, including atypical absence seizures. Alert: Closely monitor all patients taking or starting AEDs for changes in behavior indicating worsening of suicidal thoughts or behavior or depression. • Obtain baseline determinations of urinalysis, renal function, iron levels, electrolyte levels, liver function, CBC, and platelet and reticulocyte counts. • Never stop drug suddenly when treating seizures. • Adverse reactions may be minimized by increasing dosages gradually. • Monitor level and effects closely. Ask patient when last dose was taken to better evaluate drug level. Alert: Watch for signs of anorexia or subtle appetite changes, which may indicate excessive drug level. • Look alike–sound alike: Don’t confuse carbamazepine with oxcarbazepine. Don’t confuse Tegretol or Tegretol-XR with Topamax, Toprol-XL, or Toradol. Don’t confuse Carbatrol with carvedilol. CENTRAL NERVOUS SYSTEM (CNS) DRUGS Drug Class Drug Sample Pharmacodynamics Thought to be linked to drug’s inhibition of CNS neuronal uptake of serotonin. Fluoxetine Selective Serotonin Reuptake Inhibitors (SSRI) Thought to be linked to drug’s inhibition of CNS neuronal uptake of serotonin. Paroxetine ANTIDEPRESSANTS Indications Side Effects CNS: nervousness, Depression, OCD somnolence, anxiety, insomnia, Maintenance therapy for headache, drowsiness, tremor, depression in patients who dizziness, asthenia, abnormal are stabilized (not for newly thinking, fatigue, fever, diagnosed depression) emotional lability. Short-term and long-term treatment of bulimia nervosa CV: palpitations, hot flashes, Short-term treatment of panic prolonged QT interval. EENT: nasal congestion, disorder with or without pharyngitis, sinusitis. agoraphobia GI: nausea, diarrhea, dry Depressive episodes mouth, anorexia, dyspepsia, associated with bipolar I constipation, abdominal pain, disorder (with olanzapine) vomiting, flatulence, increased Premenstrual dysphoric appetite, taste perversion. disorder GU: sexual dysfunction, Treatment-resistant decreased libido, micturition depression disorder. Metabolic: weight loss, hyponatremia. Musculoskeletal: muscle pain. Respiratory: URI, cough. Skin: rash, pruritus, diaphoresis Other: flulike syndrome, chills CNS: asthenia, dizziness, Major depressive disorder headache, insomnia, (excluding Brisdelle) OCD (Paxil and Pexeva only) somnolence, tremor, nervousness, anxiety, Panic disorder (excluding paresthesia, confusion, Brisdelle) Social anxiety disorder (Paxil agitation, dysgeusia. CV: palpitations, vasodilation, and Paxil CR only) Generalized anxiety disorder HTN, tachycardia, chest pain. EENT: blurred vision, tinnitus, (Paxil and Pexeva only) lump or tightness in throat, PTSD (Paxil only) pharyngitis, rhinitis, sinusitis. Premenstrual dysphoric GI: dry mouth, nausea, disorder (PMDD) (Paxil CR constipation, diarrhea, only) flatulence, vomiting, dyspepsia, Moderate to severe decreased appetite, abdominal vasomotor symptoms pain. associated with menopause GU: ejaculatory disturbances, (Brisdelle only) sexual dysfunction, decreased Nursing Considerations Alert: If linezolid or methylene blue must be given, fluoxetine must be stopped and patient monitored for serotonin toxicity for 5 weeks or until 24 hours after final dose of linezolid or methylene blue, whichever comes first. • Use antihistamines or topical corticosteroids to treat rashes or pruritus. • Watch for weight change during therapy. • Record mood changes. Watch for suicidal tendencies. • Monitor patient for serotonin syndrome. • Monitor blood glucose level, liver and renal function. • Obtain ECG and monitor periodically in patients with risk factors for QT-interval prolongation and ventricular arrhythmia. • Monitor mental status for depression, suicidal ideation, anxiety, social functioning, mania, or panic attacks. • Observe for signs or symptoms of serotonin syndrome, akathisia, or sleep disturbances. • When discontinuing drug, taper dosage over 2 weeks to 1 month to avoid withdrawal syndrome. • Look alike–sound alike: Don’t confuse fluoxetine with fluvoxamine or fluvastatin. Don’t confuse Prozac with Proscar or Prilosec. • Patients taking Paxil CR for PMDD should be periodically reassessed to determine the need for continued treatment. • If signs or symptoms of psychosis occur or increase, expect prescriber to reduce dosage. Record mood changes. Monitor patient for suicidal tendencies. • Monitor patient for complaints of sexual dysfunction. Alert: Don’t stop drug abruptly. Alert: Combining triptans with an SSRI or an SSNRI may cause serotonin syndrome or NMS like reactions. Alert: If linezolid or methylene blue must be given, stop paroxetine and monitor patient for serotonin toxicity for 2 weeks or until 24 hrs after the last dose of methylene blue or linezolid, whichever comes first. • Look alike–sound alike: Don’t confuse paroxetine with fluoxetine or paclitaxel. Don’t confuse Paxil with Doxil, paclitaxel, Plavix, or Taxol. Thought to be linked to drug’s inhibition of CNS neuronal reuptake of serotonin. Probably linked to potentiation of serotonergic activity in the CNS resulting from inhibition of neuronal reuptake of serotonin. Depression Depression OCD Panic disorder PTSD Premenstrual dysphoric disorder Social anxiety disorder Generalized anxiety disorder Sertraline Citalopram libido, urinary frequency, other urinary disorders, dysmenorrhea, female genital tract disease. Metabolic: weight gain. Musculoskeletal: myopathy, myalgia, myasthenia, back pain. Respiratory: dyspnea. Skin: diaphoresis, rash, pruritus CNS: fatigue, headache, tremor, dizziness, insomnia, somnolence, anxiety, agitation, hyperkinesia, aggression. CV: palpitations. EENT: visual disturbances, blurred vision, mydriasis, dry mouth, epistaxis. GI: nausea, diarrhea, dyspepsia, vomiting, constipation, thirst, flatulence, anorexia, abdominal pain, decreased appetite. GU: male sexual dysfunction, decreased libido (both genders), urinary incontinence. Metabolic: weight loss. Musculoskeletal: myalgia, arthralgia. Skin: rash, pruritus, purpura diaphoresis, alopecia CNS: somnolence, insomnia, suicide attempt, anxiety, agitation, dizziness, paresthesia, migraine, impaired concentration, amnesia, depression, apathy, tremor, confusion, fatigue, fever. CV: tachycardia, orthostatic hypotension, hypotension, prolonged QT interval. EENT: rhinitis, sinusitis, abnormal accommodation. GI: dry mouth, nausea, diarrhea, anorexia, dyspepsia, • Record mood changes. Monitor patient for suicidal tendencies, and allow only a minimum supply of drug. • Assess patients for sexual dysfunction at baseline and periodically during treatment. SSRIs may cause signs and symptoms of sexual dysfunction and patients may not spontaneously report changes in sexual function. Alert: If linezolid or methylene blue must be given, stop sertraline and monitor patient for serotonin toxicity for 2 weeks or until 24 hrs after the last dose of methylene blue or linezolid, whichever comes first. Alert: Combining triptans with an SSRI or an SSNRI may cause serotonin syndrome or NMS-like reactions • Gradual dosage reduction is recommended when stopping treatment. Monitor patient for discontinuation syndrome • Look alike–sound alike: Don’t confuse sertraline with cetirizine or Soriatane. • Correct electrolyte disturbances before starting drug; monitor patients at high risk for electrolyte disturbances periodically during therapy. • Although drug hasn’t been shown to impair psychomotor performance, any psychoactive drug has the potential to impair judgment, thinking, or motor skills. • Reduce risk of overdose by limiting amount of drug available per refill. • At least 14 days should elapse between MAO inhibitor therapy and citalopram therapy. Alert: Combining triptans with an SSRI or an SSNRI may cause serotonin syndrome or NMS-like reactions Alert: If linezolid or methylene blue must be given, Unknown. May increase amount of norepinephrine, serotonin, or both in the CNS by blocking their reuptake by the presynaptic neurons. Tricyclic Antidepressants (TCA) Amitriptyline vomiting, abdominal pain, taste perversion, increased saliva, flatulence, increased appetite. GU: dysmenorrhea, amenorrhea, ejaculation disorder, erectile dysfunction, anorgasmia, polyuria, decreased libido. Metabolic: decreased or increased weight. Musculoskeletal: arthralgia, myalgia. Respiratory: URI, coughing. Skin: rash, pruritus. Other: increased sweating, yawning, decreased libido. CNS: stroke, seizures, coma, Depression (outpatients) Depression (patients who are ataxia, tremor, peripheral neuropathy, anxiety, insomnia, hospitalized) restlessness, drowsiness, dizziness, weakness, fatigue, headache, extrapyramidal reactions, hallucinations, delusions, disorientation. CV: orthostatic hypotension, tachycardia, heart block, arrhythmias, MI, ECG changes, HTN, edema, palpitations, syncope. EENT: blurred vision, mydriasis, increased IOP, tinnitus. GI: dry mouth, nausea, vomiting, anorexia, epigastric pain, diarrhea, constipation, paralytic ileus. GU: urine retention, altered libido, erectile dysfunction. Hematologic: agranulocytosis, thrombocytopenia, leukopenia, eosinophilia. Metabolic: hypoglycemia, hyperglycemia. Skin: rash, urticaria, alopecia, photosensitivity reactions, diaphoresis. stop drug and monitor patient for serotonin toxicity for 2 weeks, or until 24 hours after the last dose of methylene blue or linezolid, whichever comes first. • Don’t discontinue drug abruptly because a discontinuation syndrome can develop • Look alike–sound alike: Don’t confuse CeleXA with Zyprexa, CeleBREX, or Cerebyx. • Amitriptyline has strong anticholinergic effects and is one of the most sedating TCAs. Anticholinergic effects have rapid onset even though therapeutic effect is delayed for weeks. • Older adults may have an increased sensitivity to anticholinergic effects of drug; sedating effects of drug may increase the risk of falls in this population. • If signs or symptoms of psychosis occur or increase, expect prescriber to reduce dosage. Record mood changes. Monitor patient for suicidal tendencies and allow only minimum supply of drug. • Because patients using TCAs may suffer hypertensive episodes and arrhythmias during surgery, stop drug gradually several days before surgery. • Monitor glucose level. • Watch for nausea, headache, and malaise after abrupt withdrawal of long-term therapy; these symptoms don’t indicate addiction. • Don’t withdraw drug abruptly. • Look alike–sound alike: Don’t confuse amitriptyline with nortriptyline or aminophylline. Don’t confuse Elavil with Eldepryl or enalapril. Unknown. Increases norepinephrine, serotonin, or both in the CNS by blocking their reuptake by the presynaptic neurons. Mechanism for enuresis unknown but thought to be separate from drug’s antidepressant effects. Depression Childhood enuresis Inhibits the enzyme monoamine oxidase, resulting in an accumulation of various neurotransmitters (dopamine, epinephrine, norepinephrine, and serotonin) in the body. Treatment of major depressive episode without melancholia (usually reserved for patients who do not tolerate or respond to other modes of therapy) Imipramine Mono Amine Oxidase Inhibitors ( MAOI’s) Tranylcypromine (Parnate) CNS: drowsiness, dizziness, seizures, stroke, excitation, tremor, confusion, hallucinations, anxiety, ataxia, fatigue, peripheral neuropathy, restlessness, headache, paresthesia, nervousness, extrapyramidal reactions, agitation, sleep disorders, tiredness. CV: orthostatic hypotension, tachycardia, ECG changes, MI, arrhythmias, heart block, HTN, precipitation of HF, palpitations. EENT: blurred vision, mydriasis, tinnitus. GI: dry mouth, constipation, nausea, vomiting, anorexia, paralytic ileus, abdominal cramps, black tongue, diarrhea. GU: urine retention, urinary frequency. Hematologic: bone marrow depression, thrombocytopenia. Metabolic: hypoglycemia, hyperglycemia, weight loss, weight gain. Skin: rash, urticaria, photosensitivity reactions, pruritus, diaphoresis, alopecia. CNS: seizures, confusion, dizziness, drowsiness, headache, insomnia, restlessness, tremor, paresthesia, weakness. EENT: blurred vision, tinnitus. CV: hypertensive crisis, edema, orthostatic hypotension, tachycardia. GI: abdominal pain, anorexia, constipation, diarrhea, dry mouth, hepatitis, nausea. GU: sexual dysfunction, urinary retention. Hematologic: agranulocytosis, leukopenia, thrombocytopenia. • Monitor WBC count during therapy, and monitor patient for fever and sore throat. Discontinue drug if pathologic neutrophil depression occurs. • Monitor patient for nausea, headache, and malaise after abrupt withdrawal of long-term therapy; these symptoms don’t indicate addiction. • Safety of long-term use as adjunctive therapy for nocturnal enuresis in children age 6 or older hasn’t been established. Consider a drug-free period after an adequate therapeutic trial with a favorable response. • Don’t withdraw drug abruptly. • Because of hypertensive episodes during surgery in patients receiving TCAs, stop drug gradually several days before surgery. • If signs or symptoms of psychosis occur or increase, expect prescriber to reduce dosage. Monitor mood changes. Monitor patient for suicidal tendencies, and allow only a minimum supply of drug. • To prevent relapse in children receiving drug for enuresis, withdraw drug gradually. • Recommend sugarless hard candy or gum to relieve dry mouth. Saliva substitutes may be useful. Alert: Tofranil may contain tartrazine. • Look alike–sound alike: Don’t confuse imipramine with desipramine. Be alert for new seizures or increased seizure activity, especially at the onset of drug treatment. Document the number, duration, and severity of seizures, and report these findings to the physician immediately. Measure BP periodically & compare to normal values. Immediately report a large, rapid increase in BP. Watch for signs of agranulocytosis and leukopenia and thrombocytopenia. Be alert for increased depression and suicidal thoughts and ideology. Be alert for restlessness, confusion, or other alterations in mood and behaviour. Assess BP when patient assumes a more upright position. Skin: alopecia, rashes. MS: muscle spasm. Isocarboxazid (Marpan) Phenelzine (Nardil) Inhibits the enzyme monoamine oxidase, resulting in an accumulation of various neurotransmitters (dopamine, epinephrine, norepinephrine, and serotonin) in the body. Treatment of depression Inhibits the enzyme monoamine oxidase, resulting in an accumulation of various neurotransmitters (dopamine, epinephrine, norepinephrine, and serotonin) in the body. Treatment of neurotic or (usually reserved for patients who do not tolerate or respond to other modes of therapy) atypical depression (usually reserved for patients who do not tolerate or respond to other modes of therapy CNS: seizures, dizziness, headache, akathisia, anxiety, ataxia, drowsiness, euphoria, insomnia, restlessness, weakness. EENT: blurred vision. CV: hypertensive crisis, orthostatic hypotension. GI: nausea, black tongue, constipation, diarrhea, dry mouth. GU: dysuria, sexual dysfunction, urinary incontinence, urinary retention. Skin: photosensitivity. CNS: seizures, dizziness, drowsiness, fatigue, headache, hyperreflexia, insomnia, tremor, twitching, weakness, euphoria, paresthesia, restlessness. EENT: blurred vision, glaucoma, nystagmus. CV: hypertensive crisis, edema, orthostatic hypotension. GI: constipation, dry mouth, abdominal pain, liver function test elevation, nausea, vomiting. GU: sexual dysfunction, urinary retention. Skin: pruritus, rashes. Endo: weight gain. Assess peripheral edema using girth measurements, volume displacement & measurement of pitting edema. Assess paresthesias, tremor, or muscle spasms. Be alert for new seizures or increased seizure activity. Document the number, duration, and severity of seizures, and report these findings to the physician immediately. Measure BP periodically & compare to normal values. Immediately report a large, rapid increase in BP. Assess BP when patient assumes a more upright position. Be alert for increased depression and suicidal thoughts and ideology. Assess dizziness, drowsiness, and ataxia that might affect gait, balance, and other functional activities. Be alert for new seizures or increased seizure activity, especially at the onset of drug treatment. Document the number, duration, and severity of seizures, and report these findings immediately to the physician. Measure BP periodically & compare to normal values. Immediately report a large, rapid increase in BP. Be alert for increased depression and suicidal thoughts and ideology. Be alert for anxiety, euphoria, severe restlessness, or other alterations in mental status. Assess BP when patient assumes a more upright position. Assess peripheral edema using girth measurements, volume displacement, and measurement of pitting edema. Assess paresthesias, tremor, or increased reflex activity. Assess dizziness and drowsiness that might affect gait, balance, and other functional activities. CENTRAL NERVOUS SYSTEM (CNS) DRUGS Drug Class Drug Sample Pharmacodynamics Alters the effects of dopamine in the CNS. Has significant anticholinergic/alphaadrenergic blocking activity. Chlorpromazine Antipsychotics A. Typical Antipsychotic A butyrophenone that probably exerts antipsychotic effects by blocking postsynaptic dopamine receptors in the brain. Haloperidol PSYCHOTHERAPEUTIC DRUGS Indications Side Effects CNS: neuroleptic malignant Psychosis, mania syndrome, sedation, Nausea and vomiting extrapyramidal reactions, Acute intermittent porphyria, tardive dyskinesia intractable hiccups EENT: blurred vision, dry eyes, Tetanus lens opacities Behavioral disorders, CV: hypotension, tachycardia. hyperactivity GI: constipation, dry mouth, Preoperative sedation, anorexia, hepatitis, ileus, anxiety priapism Second-line treatment for schizophrenia and psychoses GU: urinary retention Skin: photosensitivity, pigment after failure with atypical changes, rashes antipsychotics Endo: galactorrhea, amenorrhea Hematologic: agranulocytosis, leukopenia Metabolic: hyperthermia Others: allergic reactions Schizophrenia Chronic schizophrenia requiring prolonged therapy Nonpsychotic behavior disorders Tourette syndrome CNS: severe extrapyramidal reactions, dystonia, tardive dyskinesia, NMS, seizures, sedation, drowsiness, lethargy, headache, insomnia, confusion, vertigo, agitation, anxiety, depression, euphoria, restlessness, tonic-clonic seizures, hallucinations, parkinsonian-like syndrome. CV: tachycardia, hypotension, HTN, prolonged QT interval and other ECG changes, torsades de pointes GI: dry mouth, anorexia, constipation, diarrhea, nausea, vomiting, dyspepsia. Hematologic: leukopenia, leukocytosis. Hepatic: jaundice. Metabolic: hyperglycemia, hypoglycemia, hyponatremia. Nursing Considerations Monitor and report signs of neuroleptic malignant syndrome. Watch for signs of agranulocytosis and leukopenia. Assess motor function, and be alert for extrapyramidal symptoms. Assess BP periodically and compare to normal values. Report low BP, especially if patient experiences dizziness or syncope. Assess heart rate, ECG, and heart sounds, especially during exercise. Report a rapid HR or symptoms of other arrhythmias. Monitor signs of allergic reactions. If used to control behavioral problems in children, document any changes in combative or hyperactive behavior to help determine drug efficacy and appropriate dosing. If used to control vascular headache, monitor the frequency, severity, and duration of attacks to help document the effects of drug therapy. • Monitor patient for tardive dyskinesia, which may occur after prolonged use. Alert: Watch for signs and symptoms of NMS, which is rare but commonly fatal. Alert: Monitor ECG when drug is given in high doses or when patient is taking other QT interval– prolonging drugs because of the increased risk of QT-interval prolongation and torsades de pointes. • Don’t withdraw drug abruptly unless required by severe adverse reactions. • Complete fall risk assessments at start of antipsychotic treatment and recurrently for patients on long-term therapy, especially those at increased risk for falls. • Esophageal dysmotility and aspiration can occur. Use cautiously in patients at risk for aspiration. • Look alike–sound alike: Don’t confuse Haldol with Halcion or Halog. Alters the effects of dopamine in the CNS. Has anticholinergic and alpha-adrenergic blocking activity. Acute and chronic psychotic disorders Fluphenazine Unknown. Binds Schizophrenia in patients selectively to who are severely ill and dopaminergic unresponsive to other receptors in the CNS therapies; to reduce risk of and may interfere with recurrent suicidal behavior in adrenergic, cholinergic, schizophrenia or histaminergic, and schizoaffective disorder serotonergic receptors. Antipsychotics B. Atypical Antipsychotics Clozapine CNS: neuroleptic malignant syndrome, extrapyramidal reactions, sedation, tardive dyskinesia. EENT: blurred vision, dry eyes. CV: hypertension, hypotension, tachycardia. GI: anorexia, constipation, drug-induced hepatitis, dry mouth, ileus, nausea, weight gain. GU: urinary retention. Skin: photosensitivity, pigment changes, rashes. Endo: galactorrhea. Hemat: agranulocytosis, leukopenia, thrombocytopenia. Misc: allergic reactions. CNS: drowsiness, sedation, dizziness, vertigo, headache, seizures, syncope, tremor, disturbed sleep or nightmares, restlessness, hypokinesia or akinesia, agitation, rigidity, akathisia, confusion, fatigue, insomnia, hyperkinesia, weakness, lethargy, ataxia, slurred speech, depression, myoclonus, anxiety, fever. CV: tachycardia, hypotension, HTN, chest pain, ECG changes, orthostatic hypotension EENT: visual disturbances GI: constipation, excessive salivation, dry mouth, nausea, vomiting, heartburn, diarrhea. Hematologic: leukopenia, neutropenia, eosinophilia. Metabolic: hyperglycemia, weight gain, hypercholesterolemia, hypertriglyceridemia. Respiratory: respiratory arrest. Monitor and report signs of neuroleptic malignant syndrome. Monitor signs of agranulocytosis and leukopenia or thrombocytopenia. Assess motor function, and be alert for extrapyramidal symptoms. Monitor signs of allergic reactions. Assess BP and compare to normal values. Assess heart rate, ECG, and heart sounds, especially during exercise. Periodically assess body weight and other anthropometric measures. Alert: Drug may cause hyperglycemia. Monitor patients with diabetes regularly. Alert: Monitor patient for metabolic syndrome. • Monitor patient for signs and symptoms of myocarditis and cardiomyopathy. • Some patients experience transient fever with temperature higher than 100.4° F (38° C), especially in the first 3 weeks of therapy. Monitor these patients closely. Alert: Fever may be the first sign of neutropenic infection. Interrupt therapy and obtain ANC level. • If drug is to be discontinued and patient doesn’t have moderate to severe neutropenia, reduce dose gradually over 1 to 2 weeks. • When discontinuing drug, monitor patients carefully for recurrence of psychotic symptoms and symptoms related to cholinergic rebound. • Drug can cause sedation and impair cognitive and motor performance. Monitor patient carefully for CNS changes. • Complete fall risk assessments when initiating therapy and recurrently for patients on long-term therapy. • Look alike–sound alike: Don’t confuse clozapine with clonidine, clonazepam, or Klonopin. Don’t confuse Clozaril with Colazal. May block dopamine and 5receptors. Olanzapine Risperidone Schizophrenia Short-term treatment of acute manic episodes linked to bipolar I disorder Short-term treatment, with lithium or valproate, of acute mixed or manic episodes linked to bipolar I disorder Maintenance treatment of bipolar I disorder Agitation caused by schizophrenia and bipolar I mania Depressive episodes associated with bipolar I disorder Treatment-resistant depression Preventing chemotherapyassociated acute and delayed nausea or vomiting Chemotherapy-associated breakthrough nausea or vomiting Blocks dopamine, 5 Schizophrenia , and Parenteral maintenance adrenergic, and therapy for schizophrenia or histaminergic bipolar I disorder (as receptors in the monotherapy or as brain. combination therapy with lithium or valproate) Monotherapy or combination therapy with lithium or valproate for 3-week treatment of acute manic or mixed episodes from bipolar I disorder Irritability, including aggression, self-injury, and temper tantrums, associated with an autistic disorder CNS: somnolence, insomnia, parkinsonism, dizziness, NMS, suicide attempt, abnormal gait, asthenia, personality disorder, auditory hallucinations, restlessness, fatigue, akathisia, headache, tremor, articulation impairment, tardive dyskinesia, fever, extrapyramidal events (IM), hypertonia. CV: prolonged QT interval, orthostatic hypotension, tachycardia, chest pain, HTN, ecchymosis, peripheral edema, hypotension (IM). EENT: amblyopia, conjunctivitis, rhinitis, pharyngitis. GU: hematuria, metrorrhagia, urinary incontinence, UTI, amenorrhea, vaginitis, vaginal discharge. Hematologic: leukopenia. Metabolic: hyperglycemia, dyslipidemia, weight gain. CNS: akathisia, sedation, somnolence, dystonia, headache, insomnia, agitation, pain, parkinsonism, abnormal gait, ataxia, hallucination, mania, hypoesthesia, fatigue, depression CV: tachycardia, bradycardia, bundle-branch block, ECG changes, chest pain, hypotension, edema, palpitations, HTN. GU: urinary incontinence, increased urination, abnormal orgasm, decreased libido, vaginal dryness, amenorrhea, menstrual disorder, cystitis Musculoskeletal: arthralgia, myalgia, muscle rigidity, spasm Respiratory: cough, dyspnea, bronchitis, pneumonia, URI. • ODTs contain phenylalanine. • Monitor patient for abnormal body temperature regulation, especially if patient exercises, is exposed to extreme heat, takes anticholinergics, or is dehydrated. • Obtain baseline and periodic LFT results. • Monitor patient for weight gain. • Monitor patient for mental status changes, sedation, coma, or delirium. • Monitor patient for tardive dyskinesia, which may occur after prolonged use. It may not appear until months or years later and may disappear spontaneously or persist for life, despite stopping drug. • Periodically reevaluate the long-term usefulness of olanzapine. • Patient who feels dizzy or drowsy after an IM injection should remain recumbent until assessment for orthostatic hypotension and bradycardia can be done. Patient should rest until the feeling passes. • Taper dosage slowly when discontinuing. • Look alike–sound alike: Don’t confuse olanzapine with olsalazine or quetiapine. Alert: Obtain baseline BP measurements before starting therapy, and monitor BP regularly. Watch for orthostatic hypotension. Alert: Watch for evidence of NMS. • Life-threatening hyperglycemia may occur in patients taking atypical antipsychotics. Monitor patients with diabetes regularly. Alert: Monitor patient for symptoms of metabolic syndrome. • Periodically reevaluate drug’s risks and benefits • Monitor patient for weight gain. • Monitor CBC in patients with preexisting low WBC count or history of drug-induced leukopenia or neutropenia. • Complete fall risk assessments when initiating treatment and recurrently for patients on long term therapy • Look alike–sound alike: Don’t confuse risperidone with reserpine or ropinirole. Probably alters Acute mania in bipolar chemical transmitters disorder in the CNS, possibly by Long-term control in bipolar interfering with ionic disorder pump mechanisms in brain cells, and may compete with or replace sodium ions. Antimanic Lithium Releases nerve terminal stores of norepinephrine, promoting nerve impulse transmission. At high doses, effects are mediated by dopamine. CNS Stimulants Methylphenidate ADHD Narcolepsy CNS: fatigue, lethargy, coma, seizures, tremors, drowsiness, headache, restlessness, dizziness, psychomotor retardation, blackouts, EEG changes, impaired speech, ataxia, incoordination. CV: arrhythmias, bradycardia, reversible ECG changes, severe bradycardia, hypotension, Brugada syndrome. GI: vomiting, anorexia, diarrhea, thirst, nausea, metallic taste, dry mouth, abdominal pain, flatulence, indigestion. GU: polyuria, renal toxicity with long-term use, glycosuria, decreased CrCl, albuminuria, erectile dysfunction. Hematologic: leukocytosis. Metabolic: transient hyperglycemia, goiter, hypothyroidism, hyponatremia. CNS: nervousness, headache, insomnia, seizures, tics, dizziness, akathisia, dyskinesia, drowsiness, mood swings, anxiety, irritability, depression, tremor, vertigo, confusion, insomnia, sedation, CV: palpitations, tachycardia, arrhythmias, HTN. EENT: blurred vision, eye pain, pharyngitis, sinusitis, bruxism, xerostomia, oropharyngeal pain. GI: nausea, abdominal pain, anorexia, decreased appetite, dry mouth, vomiting, constipation, dyspepsia, diarrhea. GU: decreased libido. Metabolic: weight loss. • Monitor patient and discontinue drug for signs and symptoms of lithium toxicity. • Monitor baseline ECG, thyroid studies, renal studies, and electrolyte levels. • Check fluid intake and output, especially when surgery is scheduled. • Weigh patient daily; check for edema or sudden weight gain. • Adjust fluid and salt ingestion to compensate if excessive loss occurs from protracted diaphoresis or diarrhea. • Check urine specific gravity and report level below 1.005, which may indicate diabetes insipidus. • Monitor glucose level closely. • Perform outpatient follow-up of thyroid and renal function every 2 to 3 months during 6 months of treatment, then as clinically indicated. Monitor CBC and ECG (patients older than age 40) before therapy and as needed. Monitor lithium level twice weekly, 12 hours after the last oral dose, until patient and levels are stable, then every 1 to 2 months or as needed. • Palpate thyroid to check for enlargement. Monitor weight before and during therapy. • Look alike–sound alike: Don’t confuse lithium carbonate with lanthanum carbonate. • Don’t use drug to prevent fatigue or treat severe depression. • Before starting drug, assess for the presence of cardiac disease by performing a careful history, family history of sudden death or ventricular arrhythmia, and physical exam. • Observe patient for signs of excessive stimulation. Monitor BP. • Check CBC, differential, and platelet counts with long-term use, particularly if patient shows signs or symptoms of hematologic toxicity. • Monitor height and weight in children on long-term therapy. • Monitor patient for tolerance or psychological dependence. • Carefully observe patient for digital changes. • Monitor patients using patch for chemical leukoderma. Report skin changes to prescriber. • Look alike–sound alike: Don’t confuse methylphenidate with methadone. CENTRAL NERVOUS SYSTEM (CNS) DRUGS Drug Class Drug Sample PANCURONIUM Pharmacodynamics Prevents acetylcholine from binding to receptors on the motor end plate, blocking neuromuscular transmission. ATRACURIUM Prevents neuromuscular transmission by blocking the effect of acetylcholine at the myoneural junction. NONDEPOLARIZING NMJ BLOCKERS Binds with a high affinity to cholinergic receptors, prolonging depolarization of the motor end plate and ultimately producing muscle paralysis. DEPOLARIZING NMJ BLOCKERS SUCCINYLCHOLINE NEUROMUSCULAR BLOCKERS (NMJ BLOCKERS) Indications Side Effects CV: tachycardia, increased BP, Adjunct to anesthesia to flushing. relax skeletal muscle, EENT: excessive salivation. facilitate intubation, and Musculoskeletal: residual assist with mechanical muscle weakness. ventilation Respiratory: prolonged respiratory insufficiency or apnea. Skin: transient rashes. Other: allergic or idiosyncratic hypersensitivity reactions. Respiratory: bronchospasm Induction of skeletal muscle CV: hypotension, tachycardia paralysis and facilitation of Skin: flushing, rash intubation after induction of Other: allergic reactions anesthesia in surgical including anaphylaxis procedures. Facilitation of compliance during mechanical ventilation. Adjunct to anesthesia to facilitate tracheal intubation; to provide skeletal muscle relaxation during surgery or mechanical ventilation CV: arrhythmias, bradycardia, cardiac arrest, tachycardia, HTN, hypotension, flushing. EENT: increased IOP, excessive salivation. Metabolic: hyperkalemia. Musculoskeletal: postoperative muscle pain, muscle fasciculation, jaw rigidity, rhabdomyolysis with acute renal failure. Respiratory: apnea, bronchoconstriction, prolonged respiratory depression. Skin: rash. Other: allergic or idiosyncratic hypersensitivity reactions, anaphylaxis, malignant hyperthermia. Nursing Considerations • Allow succinylcholine effects to subside before giving this drug. • Monitor baseline electrolyte determinations and VS. • Measure fluid intake and output. • Monitor respirations closely until patient recovers fully from neuromuscular blockade, as indicated by tests of muscle strength. • After spontaneous recovery starts, neuromuscular blockade may be reversed with an anticholinesterase. • Give analgesics for pain. Alert: Careful dosage calculation is essential. Assess respiratory status continuously. Monitor neuromuscular response with a peripheral nerve stimulator intraoperatively. Monitor ECG, heart rate, and BP throughout administration. Observe the patient for residual muscle weakness and respiratory distress during the recovery period. Monitor infusion site frequently. If signs of tissue irritation or extravasation occur, discontinue and restart in another vein. • Dosage depends on anesthetic used, individual needs, and response. Recommended dosages must be individually adjusted. • Monitor baseline electrolyte determinations and vital signs. Check respirations every 5 to 10 minutes during infusion. • Monitor respirations closely until tests of muscle strength. Alert: Don’t use reversing drugs. • Repeated or continuous infusions aren’t advisable; they may cause reduced response or prolonged muscle relaxation and apnea. • Give analgesics for pain. • Keep airway clear. Have emergency respiratory support equipment immediately available. Alert: Careful dosage calculation is essential. Always verify dosage with another health care professional. CENTRAL NERVOUS SYSTEM (CNS) DRUGS Drug Class Drug Sample PANCURONIUM Pharmacodynamics Prevents acetylcholine from binding to receptors on the motor end plate, blocking neuromuscular transmission. ATRACURIUM Prevents neuromuscular transmission by blocking the effect of acetylcholine at the myoneural junction. CENTRALLY ACTING MUSCLE RELAXANTS DIRECT ACTING MUSCLE RELAXANTS DANTROLENE Acts directly on skeletal muscle, causing relaxation by decreasing calcium release from sarcoplasmic reticulum in muscle cells. Prevents intense catabolic process associated with malignant hyperthermia. MUSCLE RELAXANTS Indications Side Effects CV: tachycardia, increased BP, Adjunct to anesthesia to flushing. relax skeletal muscle, EENT: excessive salivation. facilitate intubation, and Musculoskeletal: residual assist with mechanical muscle weakness. ventilation Respiratory: prolonged respiratory insufficiency or apnea. Skin: transient rashes. Other: allergic or idiosyncratic hypersensitivity reactions. Respiratory: bronchospasm Induction of skeletal muscle CV: hypotension, tachycardia paralysis and facilitation of Skin: flushing, rash intubation after induction of Other: allergic reactions anesthesia in surgical including anaphylaxis procedures. Facilitation of compliance during mechanical ventilation. Spasticity and sequelae from severe chronic disorders, such as MS, cerebral palsy, spinal cord injury, and stroke To manage malignant hyperthermic crisis To prevent or attenuate malignant hyperthermic crisis in susceptible patients who need surgery To prevent recurrence of malignant hyperthermic crisis CNS: drowsiness, muscle weakness, confusion, dizziness, headache, insomnia, malaise, nervousness. EENT: excessive lacrimation, visual disturbances. Respiratory: pleural effusions. CV: changes in blood pressure, tachycardia. GI: hepatotoxicity, diarrhea, anorexia, cramps, dysphagia, GI bleeding, vomiting. GU: crystalluria, dysuria, frequency, erectile dysfunction, incontinence, nocturia. Skin: pruritus, sweating, urticaria. Hematologic: eosinophilia. Musculoskeletal: myalgia. Nursing Considerations • Allow succinylcholine effects to subside before giving this drug. • Monitor baseline electrolyte determinations and VS. • Measure fluid intake and output. • Monitor respirations closely until patient recovers fully from neuromuscular blockade, as indicated by tests of muscle strength. • After spontaneous recovery starts, neuromuscular blockade may be reversed with an anticholinesterase. • Give analgesics for pain. Alert: Careful dosage calculation is essential. Assess respiratory status continuously. Monitor neuromuscular response with a peripheral nerve stimulator intraoperatively. Monitor ECG, heart rate, and BP throughout administration. Observe the patient for residual muscle weakness and respiratory distress during the recovery period. Monitor infusion site frequently. If signs of tissue irritation or extravasation occur, discontinue and restart in another vein. Be alert for signs of hepatotoxicity Report these signs to the physician immediately. Assess patient's spasticity, ROM, functional ability, and posture, especially when beginning Dantrium treatment or during dose adjustments. Assess dizziness or drowsiness that might affect gait, balance, and other functional activities. Monitor symptoms such as confusion, nervousness, insomnia, or malaise. Monitor signs of eosinophilia. Assess BP and compare to normal values. Assess heart rate, ECG, and heart sounds, especially during exercise. Assess any breathing problems or signs of pleural effusion. Assess injection site during and after IV administration, and report signs of phlebitis. BOTULINIUM TOXIN A BOTULINIUM TOXIN B Produces partial chemical denervation by inhibiting the release of acetylcholine. Result is local decrease in muscle activity. Temporary improvement in Binds to and cleaves the synaptic Vesicle Associated Membrane Protein, which is a component of the protein complex responsible for docking and fusion of the synaptic vesicle to the presynaptic membrane, a necessary step to neurotransmitter release. Treatment of patients with the appearance of moderateto-severe glabellar lines associated with corrugator and/or procerus muscle activity in adults ≤65 yr. cervical dystonia to reduce the severity of abnormal head position and neck pain associated with cervical dystonia. CNS: headache. EENT: temporary eyelid droop. GI: nausea. Local: discomfort at injection sites. Musculoskeletal: local muscle weakness. Other: allergic reactions, including anaphylaxis (rare). CV: heart burn EENT: blurry vision, accommodation difficulties, conjunctival irritation, dry nasal mucosa GI: indigestion, dry mouth, severe dry throat associated with dysphagia, swallowing difficulties, constipation, head instability, thrush Other: reduced sweating Monitor signs of allergic reactions and anaphylaxis. Notify physician immediately if these reactions occur. Be alert for possible systemic effects that might occur if botulinum toxin spreads beyond the IM injection site. Report signs of systemic botulism to the physician immediately. Assess spasticity, ROM, and functional ability as the drug begins to take effect. Monitor IM injection site for redness and irritation. Assess for mentioned contraindications and cautions to prevent untoward complications. Conduct thorough physical assessment to obtain baseline data. Monitor liver and renal function tests to detect potential adverse effects.