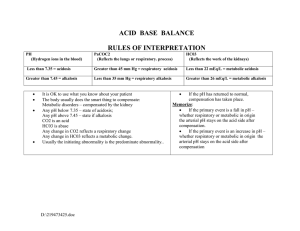
NCM 112 Module ______________________________________________________________________ FLUIDS, ELECTROLYTES, AND ACID-BASE IMBALANCES 2.2 Acid-Base Imbalances ______________________________________________________________________ Elinor B. Regondola, RN, MAN COLLEGE OF NURSING ACID-BASE IMBALANCES IMPORTANCE OF ACID-BASE BALANCE Lesson 1 INTRODUCTION To maintain homeostasis, the human body employs many physiological adaptations. One of these is maintaining an acid-base balance, in the absence of pathological states, the pH of the human body ranges between 7.35-7.45, with the average of 7.40. Why this number? Why not a neutral number of 7.0 instead of a slightly alkaline 7.40? A pH at this level is ideal for many biological processes, one of the most important, being the oxygenation of blood Learning Objectives At the end of this lesson you should be able to: 1. Identify the different types of acid-base balance 2. Site examples with regards to the Ph concentration 3. Compare the different acid-base balances. Try This! The pH Scale Identify and write on the space provided for whether the item is an Acid or a Base and on the middle part the neutral Ph. Base And on the middle part the neutral pH. BASE ACID NEUTRAL pH Adapted from: illustrations @google.com Hydrochloric Acid Drain Cleanser Vinegar Soap Blood Water Apple Stomach acid Milk Tomato Lemon Spirit of ammonia Baking soda Banana Soft drinks Think Ahead! 1. Based on the activity, what made you think that these items are acidic in nature? (Specify the items that you consider to be acidic) Justify your answer. 2. What items are alkaline in nature? (Specify the items that you consider to be base in nature) Justify your answer. 3. List the items that were considered to be Neutral. (Specify the items that you considered to be neutral in nature) Justify your answer. 4. Ever wonder what pH stand for? If so, give the three different types of pH or potential of ion concentration within the solution. . Read and Ponder Acid- Is any proton donor (a molecule that releases a proton H+ In water). Ex. Strong acid: HCL, Weak acid: Carbonic and Lactic acids). Base – Is a proton acceptor (A substance that accepts H+ often with the release of hydroxyl (OH) ion. Ex. Strong acid: hydroxyl, Weak acid: Bicarbonate (HCO3). PH - Is another term for H+ concentration that is generally used nowadays instead of hydrogen ion concentration. An increase in the H+ ion concentration decreases the pH (Acidosis) and a reduction in the H+ concentration increases the pH (Alkalosis). In a healthy person the pH is 7.40 and varies between 7.38-7.42. A slight change in the pH below 7.38 or above 7.42 will cause serious threats to many physiological functions. See if you can do this! Do you still recall the differences in the Ph concentration of an acid, base, and a neutral pH? Name the 3 most important Ph concentration, differentiate one from the other. ACID-BASE IMBALANCES Different Compensatory Mechanism Used to regulate acid-base status Lesson 2 INTRODUCTION Have you ever wondered how the body regulates Acid-base Status by binding the H+ ion, eliminates carbon dioxide ( CO2 )and conserves the bases ( HCO3 ) thus maintaining the acid-Base Balance. The body has three different mechanisms to regulate acid- Base status. 1. Acid- base buffer – Is a combination of a weak acid and a base. Wherein its pH changes very little when a small amount of Strong acid or base is added. Three types of Acid-Base buffer system: a. Bicarbonate buffer system – Is present in the Extracellular fluid (Plasma) ex. HCO3, which is regulated by the kidney. b. Phosphate buffer system – Is useful in the intracellular fluid (RBC or other cells). Mostly concentrated in the ICF than in the ECF. Is useful in the tubular fluids of kidneys c. Protein buffer system – Are present in the blood; both in the plasma and electrolytes. 2. Regulation of acid-base balance by respiratory mechanism - Entire reaction is reverse in the lungs when CO2 diffuses from the blood in to the alveoli of lungs. When the metabolic Activity increases, more amount of CO2 is produced in the tissues and the concentration of H+ increases thus increasing pulmonary ventilation (Hyperventilation) thereby the excess CO2 is removed from the body. 3. Regulation of acid-base balance by renal mechanism - Kidney maintains the acid-base balance of the body by the secretion of H+ and retention of HCO3. Among the three mechanisms the acid-base buffer is the fastest one and it readjust the pH within seconds, the respiratory mechanism does it in minutes, whereas the renal mechanism is slower and it takes few hours to few days to bring the pH to normal. However, the renal mechanism is the most powerful in maintaining acid-base Balance of the body fluids. Learning Objectives At the end of this lesson you should be able to: 1. Discuss the different mechanisms involved to regulate acid-base Status 2. Compare the different acid-base buffer system 3. Identify which compensatory mechanism is the most important In regulating acid-base balance . Try This! Have you ever thought what parts or organs of your body are involved in the compensatory mechanism to regulate the acid-base status and which of these buffer system works the fastest or the slowest. Name the parts or organs that are involved in the compensatory mechanism to regulate acid-base balance and opposite to that , write which among these buffer system or mechanism works the fastest or the slowest. ( Acid-base buffer system, respiratory mechanism, Renal mechanism, and which works within seconds, minutes, hours or days ). See the diagram below. ( carries blood ) ( carries blood ) Adapted from illustrations of internal organs @ Google.com ACID-BASE BUFFER SYSTEM Organ Involved? RESPIRATORY MECHANISM Organ Involved? RENAL MECHANISM Organ Involved? How it works? How it works? How it works? Duration and Rationale? Duration and Rationale? Duration and Rationale? Think Ahead! 1. How would you describe a buffer system? 2. Can you name the three different types of buffer system under the acid-base system and differentiate one from the other. 3. How is CO2 removed from the body? 4. Which organ is responsible for the regulation of acid-base Balance by renal mechanism and how is it made possible? Read and Ponder! Respiratory Acidosis - caused by accumulation of CO2 due to pulmonary hypoventilation. Main Causes are: - Respiratory failure as a result of pulmonary diseases such as Bronchopneumonia, emphysema, asthma and COPD. Neuromuscular disease. CNS depression Certain drugs e.g. morphine and barbiturates Signs and Symptoms: * Headache * Muscle Weakness * Skin, pale/Cyanotic * Dysrhythmias * Rapids shallow respiration * Decrease BP * Hyperkalemia * Changes in LOC Respiratory Alkalosis – caused by excessive loss of CO2 as a result of hyperventilation. Main Causes are: Adult respiratory - Salicylate intoxication Head injury Hysteria Maybe due also to hyperventilation as a result of anxiety attack and response to severe pain. Signs and Symptoms: * Seizures * Numbness or Tingling Of ext. * Lightheadedness * Tachycardia * Rapids shallow respiration * Decrease or normal BP * Hypokalemia * Lethargy and confusion Metabolic Acidosis – caused by accumulation of net acid (Most encountered in the clinical practice). Main Causes are: - Ingestion of acid e.g. aspirin intoxication Excessive production of acids e.g. ketoacidosis Decrease excretion of acids e.g. renal failure Excessive loss of HCO3 e.g. diarrhea - Due to cardiac arrest and any condition associated with Hypovolemic shock (Severe blood loss) Signs and Symptoms: * Headache * Nausea, vomiting, diarrhea * Muscle twitching * Warm flushed skin * Kussmaul respiration * Decrease BP * Hyperkalemia * Confusion, drowsy Metabolic Alkalosis – caused by loss of H+ or increase in base. Main causes are: - Diuretic use causing volume depletion (K and CL) - Increase HCO3 reabsorption and increase H+ secretion - Other causes maybe due to recurrent vomiting, Dietary CL deficiency and chronic K depletion. - Occurs in patients with pyloric stenosis due to Severe projectile vomiting. Signs and Symptoms: * Compensatory hypoventilation * Nausea, vomiting, diarrhea * Tremors, muscle cramps and Tingling of fingers and toes * Hypokalemia * Tachycardia * Dizzy, irritable, Restless See if you can do this! Can you still remember the different types of acid and alkalosis? Name the different types and distinguish one from the other. ACID-BASE IMBALANCES DIFFERENT ACID-BASE IMBALANCES THEIR CAUSES AND THEIR CLINICAL SIGNS AND SYMPTOMS Lesson 3 INTRODUCTION When you breathe, your lungs removes excess Carbon dioxide from your body. When they cannot do so, your blood and other fluids becomes too acidic, or when your kidneys are unable to remove it. For now, we should be able to realize the Importance of knowing the main causes and clinical Manifestations or signs and symptoms of these acidBase imbalances. Learning Objectives At the end of the lesson you should be able to: 1. Identify the different acid-base imbalances 2. Determine the different acid-base imbalances based on their Causes and clinical signs and symptoms. 3. Interpret the different arterial blood gases results. 4. Formulate a nursing care plan using PICOT format in clinical Questioning Try This! Differentiate the four acid-base status resulting from either respiratory or metabolic factors with regards to their main causes and clinical signs and symptoms. Main Causes Respiratory Metabolic Acidosis Acidosis Signs and Symptoms Main Causes Signs and Symptoms Respiratory Alkalosis Main Causes Signs and Symptoms Main Causes Signs and Symptoms Signs and Symptoms Metabolic Alkalosis Think Ahead! 1. What are the four types of acid-base imbalance and which type is the most Common imbalance encountered in the clinical practice? 2. How does metabolic alkalosis occur? 3. Which type of acid-base imbalance causes kussmaul Respiration and what could Probably be the cause for it? 4. What causes rapid shallow respiration in respiratory acidosis? Your kidneys and lungs works to maintain acid-base Balance. Even slight variations from the normal range can have a significant effect. Read and Ponder! Acid-base balance is very important for the homeostasis of the body and almost all physiological activities depend upon the acid-base status of the body. Acids are constantly produced in the body however, acid production is balanced by the production of bases so that the acid-base status of the body is maintained. Acid-base disturbance can be determined by the Patient’s blood gases and electrolytes. Normal Values are: PH- Refers to the potential or power or hydrogen concentration within the solution. Low pH – If the pH number is lower than 7, the solution is an acid. High pH – If the pH is greater than 7, a solution is basic or alkaline. Neutral pH – If the pH is 7, then the solution is neutral. * Blood PH = 7.35-7.45 * paO2 = 80-100 mm Hg * PaCO2 (CO2 content) = 35-45 mm Hg *HCO3 (Bicarbonate content) = 22-26 mEqL *O2 Saturation = 95-100% * BE/BD (Base Excess / Base Deficit) = -2 to +2 RESPIRATORY ACIDOSIS – Occurs when breathing is Inadequate and PaCO2 builds up. RESPIRATORY ALKALOSIS – Occurs as a result of hyperventilation or excess aspirin intact. METABOLIC ACIDOSIS – In Metabolic acidosis metabolism is impaired, causing a decrease in bicarbonates and a buildup of lactic acid. METABOLIC ALKALOSIS – Occurs when bicarbonate Ion concentration increases, causing an elevation in the blood pH. ABG ANALYSIS ( arterial blood gas ) pH RESPIRATORY ACIDOSIS PacO2 pH RESPIRATORY ALKALOSIS PacO2 pH METABOLIC ACIDOSIS HCO3 pH METABOLIC ALKALOSIS HCO3 NMENOMICS : : Better think about R- Respiratory O – Opposite M- Metabolic E – Equal NOTE : If the pCO2 is affected it is ALWAYS, RESPIRATORY If HCO3 is affected it is ALWAYS, METABOLIC UNCOMPENSATED: Co2 or HCo3 normal results. PARTIALLY COMPENSATED: Nothing is normal COMPENSATED: pH is normal (7-4 baseline/neutral Courtesy of : * https://acutecare testing.org. STEPS IN ABG ANALYSIS USING THE TIC-TAC-TOE METHOD: 1. Memorize the normal values. 2. Determine if the pH is NORMAL, ACIDOSIS Or ALKALOSIS. 3. Determine if the PaCO2 is under NORMAL, ACIDOSIS or ALKALOSIS. 4. Determine if the HCO3 is under NORMAL, ACIDOSIS, or ALKALOSIS. 5. Determine if the values interpret: ACIDOSIS Or ALKALOSIS. 6. Determine if the values define: METABOLIC Or RESPIRATORY. 7. Lastly, determine the compensation if it is: FULLY COMPENSATED, PARTIALLY COMPENSATED or UNCOMPENSATED.