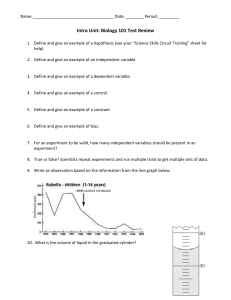
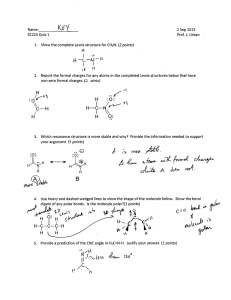
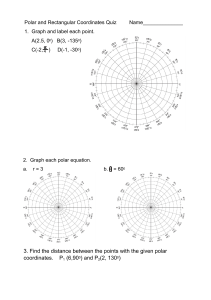
Emsal Miray Akşit 9.11.2022 21902521 Experiment 5: Thin-Layer Chromatography Report Purpose There are several purposes of this experiment. The first one is to determine the number of components in a mixture. This helps to determine relative amount of the substances and planning further experiments. Another purpose of Thin-Layer Chromatography is to identify of two substances. And also, monitoring the progress of a reaction is the uses of TLC. It provides that watching reactants disappear and products appear by using TLC. To determine the effectiveness of a purification, extraction, distillation, etc is also can be monitored by using TLC. Even though, TLC method is appropriate for microscopic compounds, adsorbents are commonly used for TLC. Introduction Thin-Layer Chromatography technique is fast, sensitive, simple and inexpensive analytical technique that is used for organic experiments. It is usually used for microscope size substances. They are 10-9 g of material. There are several uses of TLC as written above. They are determining the number of components in a mixture, identifying of two substances, monitoring the progress of a reaction, determining the effectiveness of purification, and so on. Alumnia is the most common used for adsorbed for counting chromatography. Since alumnia adsorb the substances more strongly, it is used for non-polar substances such as ketones, hydrocarbons, alkyl halides and ethers. In order to separate more polar substances like alcohols, carboxylic acids,etc, silica gel that is less adsorbent is used. In this experiment, colourless compounds are used that are Anthracene, 1-Naphthaldehyde, 1-Naphthol. And expected to find an optimal solvent system by which these compounds are seperable and to measure the Rf value. The thin layer technique is applied for these colorless compounds. The spots might be visualized under an ultraviolet light if the plates are coated with a fluorescent indicator. During the process, spots appear rapidly and disappear rapidly so, outlined each spot with a pencil immediately. This method is used mainly to isolate non-volatile mixtures. Each component is seen as spots separated vertically. Rf factor is expressed as distance travelled by sample / distance travelled by solvent. There are several factors that affect the Rf. These are solvent system, amount of material spotted, adsorbent and temperature. The reason of why TLC is used commonly is because it one of the least expensive fastest and simplest chromatography technique. The type of adsorbents is important because when adsorbent is more strongly, less time it will spend in the mobile phase. If the substance that is used for chromatography is coloured, components can be seen visually. Since in this experiment, colorless compounds will be used, then it is possible to detect compounds. These colorless compounds could be detected by placing the dry TLC plate in a jar that contains a few crystals of iodine. There is an important point that during the technique, spots appear rapidly so, outlining each spot with a pencil immediately has crucial role. They also disappear rapidly. Cleaning up and waste disposal Solvents should be placed in the organic solvents waste container and dry. Chromatographic plates can be discarded in the nonhazardous solid waste container. Observation Thin-layer chromatography is a technique is used to isolate non-volatile mixtures such as Anthracene, 1-Naphthaldehyde, 1-Naphthol were used. This experiment is conducted on a aluminium foil sheet or glass which is coated with a thin layer absorbent. This material usually is used is aluminium oxide, cellulose, silica gel. The reason why TLC is used commonly is because it is fastest, least expensive and easiest chromatography technique. In our lab we used sheet of aluminium foil to conduct the experiment. Sheet of aluminium foil was cut according the measurement written on the board. Five sheet of aluminium foil were used to chromatography. Also, three tubes were used to mix the solvents. The mobile phases are %100 hexane, %100 Ethyl Acetate, 1:1 Ethyl Acetate and Hexane, 1:3 Ethyl Acetate and hexane, 1:9 Ethyl Acetate and Hexane. The fifth of the mixture contains all of the spots; Anthracene 1-Naphtol, 1-Naphtaldehyde. All of the mixtures dissolve in excess DCM. When DCM was added to 1-Naphtol, solution turned pink colour and 1-Naphtaldehyde turned yellow and finally, Anthracene turned to white and it is like particles. Results and discussion First of all, our group had to try this experiment three times since we did the same mistakes two times. Until the third trial we could not obtain the expected result. Since the Anthracene is non-polar molecule and it has no polar group, it has to move mobile phase. 1Naphtaldehyde is a polar molecule so, it does not move with mobile phase. 1-Naphtol is a polar molecule and it is as the 1-Naphtaldehyde. The three spots are written above and the fourth spot contained all of the spots. For sheet II, anthracene a non-polar molecule and the mobile phase is %100 Ethyl Acetate and very polar, so anthracene will move up. 1Naphthaldehyde is a polar molecule and the mobile phase is %100 Ethyl Acetate and very polar, so 1-Naphthaldehyde will also go up. 1-Naphthol is also a polar molecule and the mobile phase is %100 Ethyl Acetate and polar, so 1-Naphthol moves up. Spot 8 contains all 4 spots. It could be easily said that the spot has a horizontal same level if we compared with the other spots on the TLC sheet. It showed that compounds could not be separated and move up. In the sheet III, Anthracene is a non-polar molecule. In this spot there was an error due to excess amount of compound on the TLC sheet.1-Naphthaldehyde is a polar molecule. Since the mobile phase is %50 Ethyl Acetate and %50 Hexane the mobile phase is expected that more polar than sheet 1. Therefore, 1-Naphthaldehyde goes up. However, it is not as far as the sheet 2. 1-Naphthol is a polar molecule and the mobile phase is %50 Ethyl Acetate and %50 Hexane. The mobile phase is more polar than sheet 1’s mobile phase. 1-Naphthol moves up but not as far as the like in sheet 2.the spot has a same when we compared to other spots on the TLC sheet. It can be interpreted that the less droplets of Anhracene , the less dispersion occurs. Anthracene is a non-polar molecule. The mobile phase of it is %10 Ethyl Acetate and %90 Hexane. The mobile phase of it is more polar than sheet iii’s mobile phase but not as polar as in sheet ii. Our expectations and results are different so, it could be count as an error. The reason of that error is might be due to too much solvent. In the first and second trial we might pour too much solvent into the beaker and to the alumina folder so, our results did not give an expected conclusion. Another reason of that error is might be due to experimenters’ error. While we were pouring the droplets of the mixtures, we accidentally poured too much liquid into the folder. 1-Naphthaldehyde is a polar molecule and the mobile phase is %10 Ethyl Acetate and %90 Hexane. The mobile phase is more polar than sheet iii’s mobile phase but not as polar as sheet ii. Again in this part, errors occured due to experimenters’ error and probably too much solvent. 1-Naphthol is a polar molecule and the mobile phase is %10 Ethyl Acetate and %90 Hexane. The mobile phase of it is more polar than sheet iii’s mobile phase. The last spot contain all of the spots. Conclusion In this particular lab session, we conducted an experiment which was thin layerchromatography. We learned the general theory of the TLC. TLC is the mainly based on separation principle. The separation depends on relative affinity of compounds. The movement occurs in such a way that the compounds which have a higher affinity to the stationary phase and move slowly but other compound moves fast. Also, if the mobile phase has non-polar character the compounds usually do not go up. According to our experiment, the 1:3 gave the best result. References Williamson, K. L., & Masters, K. M. (2017). Macroscale and Microscale Organic Experiments. Cengage Learning.