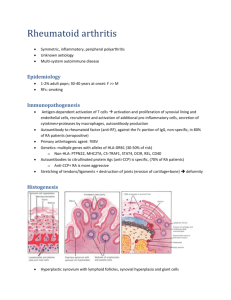
RHEUMATOID ARTHRITIS BY: DR. AYOOLA T.S INTRODUCTION/DEFINITION EPIDEMIOLOGY PATHOPHYSIOLOGY ETIOLOGY OUTLINE PRESENTATION DIAGNOSIS/DIFFERENTIALS TREATMENT COMPLICATIONS CONCLUSION Arthritis is a broad term that simply means joint inflammation. It includes swelling, pain, and stiffness of any of the joints. INTRODUCTION /DEFINITION Rheumatoid arthritis is one of the seropositive forms of arthritis and is a chronic, systemic inflammatory disease and is usually characterised by symmetric, deforming polyarthritis that affects the hands and feet. Can affect any joint lined by a synovial membrane but less commonly affects large joints. RA is theorized to develop when a genetically susceptible individual experiences an external trigger e.g. trauma, infection, that triggers an autoimmune reaction. They have a 2-3 fold increased risk of developing cardiovascular disease. Incidence: Worldwide, annual incidence rate of RA is approx. 3 cases per 10,000 population. Prevalence: Roughly 1%. Increases in smokers. EPIDEMIOLOGY Age: Affectation peaks between the ages of 35 and 50 years. Sex distribution: Women more commonly affected than men (3:1) Ethnic groups: Affects all populations, however, much more prevalent in the Native American group and less prevalent in black persons from the Caribbean region. First-degree relatives of individuals with RA are at a 2-3 fold higher risk for the disease. Concordance rate in monozygotic twins is approx. 1520%. Associated with HLA -DR4 and HLA-DR1 which serves as a pointer to disease severity. Pathogenesis not fully understood. An external trigger that sets off an autoimmune PATHOPHYSIOLOGY reaction, leading to synovial hypertrophy and chronic joint inflammation along with the potential for extraarticular manifestations, is theorised to occur in genetically susceptible individuals. The onset of clinically apparent RA is preceded by periods of pre-rheumatoid arthritis(pre-RA). Phases in the development and progression of pre-RA to RA include: • Phase I – Interaction of genetic and environmental risk factors. • Phase II – Production of RA autoantibodies such as Rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP). • Phase III – Development of arthralgia or joint stiffness without any clinical evidence of arthritis. • Phase IV – Development of arthritis in one or more joints (if intermittent, it is termed palindromic rheumatism). • Phase V – Established RA. Synovial cell hyperplasia and endothelial cell activation Uncontrollable inflammation Destruction of bones, cartilages, ligaments, tendons, blood vessels. Genetic Environmental ETIOLOGY Infectious Hormonal Immunologic Typical presentation involves symmetrical swollen, painful and stiff small joints of the hands and feet, usually worse in the morning. Affectation of the large joints (less common) PRESENTATION Less common presentations include: • Sudden-onset widespread arthritis • Recurring mono/polyarthritis of various joints (palindromic) • Persistent monoarthritis (knee, shoulder, hip) • Polymyalgic onset – vague limb girdle aches. • Recurrent soft tissue problems e.g. frozen shoulder, carpal tunnel syndrome, de Quervain’s tenosynovitis. Signs Can be classified into early and late Early signs are signs of inflammation with no joint damage e.g. swollen joint, painful joints but joint architecture is preserved. Late signs - They include: • Ulnar deviation • Subluxation of the wrist and fingers • Boutonierre (buttonhole) deformity • Swan neck deformity • Z deformity • Hand extensor tendon rupture • Atlanto-axial subluxation (rare, causing cord compression syndrome) Boutonierre’s deformity Ulnar deviation Swan neck deformity Z deformity EXTRA-ARTICULAR MANIFESTATION Affects about 40% of patients. They include: I. Nodules: Found in the olecranon process, proximal ulna, back of the heel, occiput, ischial tuberosity are common sites. II. Lungs: Pleural disease, Interstitial fibrosis, Bronchiolitis obliterans, Organising pneumonia III. CVS: IHD, Pericarditis, Pericardial effusion IV. Ocular: Episcleritis, Scleritis, Scleromalacia, Keratoconjuctivitis sicca V. Others: Osteoporosis, Carpal tunnel syndrome, peripheral neuropathy, Felty syndrome (RA, Splenomegaly, Neutropaenia) Investigative workup for patients suspected to have RA include: Laboratory studies • Serology Rheumatoid factor assay: +ve in 60-80% of patients, non-specific for RA. Predicts radiographic progression of bone erosions. Anticyclic citrullinated peptide (anti-CCP) antibody: Highly DIAGNOSIS specific (>98%) with sensitivity of about 80%. Used to predict disease progression. Anti-mutated citrullinated vimectin (anti-MCV) antibody: Comparable sensitivity with anti-CCP antibody but lower specificity Antinuclear antibody (ANA) assay: Present in about 40% of patients. • Haematology FBC: Anaemia of chronic disease, thrombocytosis Elevated ESR Elevated CRP Radiographic studies • Xray: Juxta-articular osteopaenia, soft tissue sweeling, reduced joint space. • MRI: Used primary in patients with abnormalities of the cervical spine.More accurate assessment and early detection of lesions. • Ultrasonography: Recognition of effusion and cysts, visualization of tendon sheaths, changes and degree of vascularization of synovial membrane, and erosions. According to the 2010 ACR/EULAR diagnostic criteria: Rheumatic fever SLE Degenerative joint disease DIFFERENTIALS Gouty arthritis Pyogenic arthritis Polymyalgia rheumatica Lung and gastric carcinomas, causing hypertrophic pulmonary osteoarthropathy Treatment options include: TREATMENT i. Pharmacologic therapy ii. Non-pharmacologic therapy PHARMACOLOGIC THERAPY Aimed at retarding disease progression and inducing disease remission Pharmacological agents used include: • NSAIDS e.g. Celecoxib, Diclofenac, Naproxen, Piroxicam • Corticosteroids • Disease Modifying Anti-Rheumatoid Drugs (DMARDs) DMARDs Divided into: a. Non-biologics e.g. Methotrexate, Hydroxychloroquine, Sulfasalazine, Leflunomide, Azathioprine, Cyclosporin b. Biologics a. TNF-∝ inhibitors: Etanercept, Infliximab, Adalimumab b. 𝛽-cell depleters: Rituximab c. IL-1 and IL-6 inhibitors: Tocilizumab, Anakinra d. T-cell stimulation inhibitors: Abatacept DMARDs are first-line agents but they are commenced after 3 months of NSAID use with no improvement. For early RA <6 months, administer DMARD monotherapy in atients with low-high disease activity. If disease activity remains moderate/high despite monotherapy, use combination DMARDs or a biologic agent. For established RA, combination DMARDs < anti-TNF biologic < non-TNF biologic < Tofacitinib NON-PHARMACOLOGICAL THERAPY Exercise Diet Counselling Heat and cold therapy Physiotherapy Occupational therapy Surgery Anaemia Infections GI problems Osteoporosis COMPLICATIONS Pulmonary fibrosis Ischaemic heart disease Sjogren syndrome Felty syndrome Lymphomas and other cancers. Rheumatoid arthritis is a chronic, systemic inflammatory disease and is usually characterised by symmetric, deforming polyarthritis that affects the hands and feet. Specific cause is unknown but it is theorised to be brought on by an interplay among genetic, environmental, immunologic, hormonal and infectious factors. There are articular and extra-articular manifestations of the CONCLUSION disease. Treatment options include pharmacologic and non-pharmacologic approaches. DMARDs are the mainstay of treatment. Prognosis depends largely on disease progression and effective management.