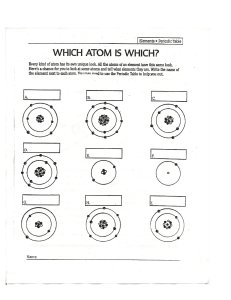
Everything around us is made up of tiny particles called atoms. What is an atom? •the word "atom" is derived from the Greek word, “atomos” or indivisible •is the smallest unit of matter that retains the identity of the substance •smallest particle of a given element Atoms are composed of three types of subatomic particles and these are the protons, electrons, and neutrons. The nucleus, which is found at the center of the atom contains protons (positively charged) and neutrons (no charge). The outermost regions of the atom contain the electrons (negatively charged). charged balloons REPULSION charged ballpen uncharged paper ATTRACTION Directions: Label the diagram by writing the correct term from the box. 1. 2. 3. 4. 5. Nucleus Proton Electron Neutron Electron shell 6. Which particle of an atom has a negative charge? 7. Which particle of the atom contains no electric charge? 8. Which particle of the atom features a positive electric charge? 9-10. Which two sub-atomic particles are located within the nucleus of an atom? st 1 Atomic Model Joseph John Thomson He discovered that atoms have negatively charged particles, which he called electrons. J.J. Thomson discovered ELECTRONS J.J. Thomson proposed the Plum Pudding Model Plum-Pudding Model After the experiment Rutherford observed the following: •Most alpha particles were undeflected. •Some are deflected at smaller angles. •Few alpha particles deflected almost back towards the source. Nuclear Model Rutherford proposed this Nuclear Model, wherein protons and neutrons were concentrated in the center of an atom and this region is called nucleus. Electrons are found outside of the nucleus and are moving along the orbits or electron shells.